Nitrone Cycloaddition on:
[Wikipedia]
[Google]
[Amazon]
 In
In
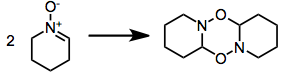
 Carbonyl condensation methods avoid issues of site selectivity associated with the oxidation of hydroxylamines with two sets of (alpha) hydrogens.
Carbonyl condensation methods avoid issues of site selectivity associated with the oxidation of hydroxylamines with two sets of (alpha) hydrogens. A significant problem associated with many reactive nitrones is dimerization. This issue is alleviated experimentally by employing an excess of the nitrone or increasing the reaction temperature to exaggerate entropic factors.
A significant problem associated with many reactive nitrones is dimerization. This issue is alleviated experimentally by employing an excess of the nitrone or increasing the reaction temperature to exaggerate entropic factors.

 In
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clay ...
, a nitrone is a functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the res ...
consisting of an ''N''-oxide of an imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bo ...
. The general structure is , where R’ is not a hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
. A nitrone is a 1,3-dipole, and is used in 1,3-dipolar cycloadditions. Other reactions of nitrones are known, including formal +3cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity". ...
s to form 6-membered rings, as well as formal +2cycloadditions to form 7-membered rings.
Generation of nitrones
Nitrones are generated most often either by the oxidation ofhydroxylamine
Hydroxylamine is an inorganic compound with the formula . The material is a white crystalline, hygroscopic compound.Greenwood and Earnshaw. ''Chemistry of the Elements.'' 2nd Edition. Reed Educational and Professional Publishing Ltd. pp. 431–43 ...
s or condensation of monosubstituted hydroxylamines with carbonyl compounds (ketones or aldehydes). The most general reagent used for the oxidation of hydroxylamines is mercury(II) oxide.
 Carbonyl condensation methods avoid issues of site selectivity associated with the oxidation of hydroxylamines with two sets of (alpha) hydrogens.
Carbonyl condensation methods avoid issues of site selectivity associated with the oxidation of hydroxylamines with two sets of (alpha) hydrogens. A significant problem associated with many reactive nitrones is dimerization. This issue is alleviated experimentally by employing an excess of the nitrone or increasing the reaction temperature to exaggerate entropic factors.
A significant problem associated with many reactive nitrones is dimerization. This issue is alleviated experimentally by employing an excess of the nitrone or increasing the reaction temperature to exaggerate entropic factors.

Reactions
1,3-dipolar cycloadditions
As 1,3-dipoles, nitrones are useful in 1,3-dipolar cycloadditions. Upon reaction of a nitrone with analkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
dipolarophile, an isoxazolidine is formed:

See also
* N-Oxoammonium salt * NitronateReferences
{{reflist Functional groups