|
Naringenin Chalcone
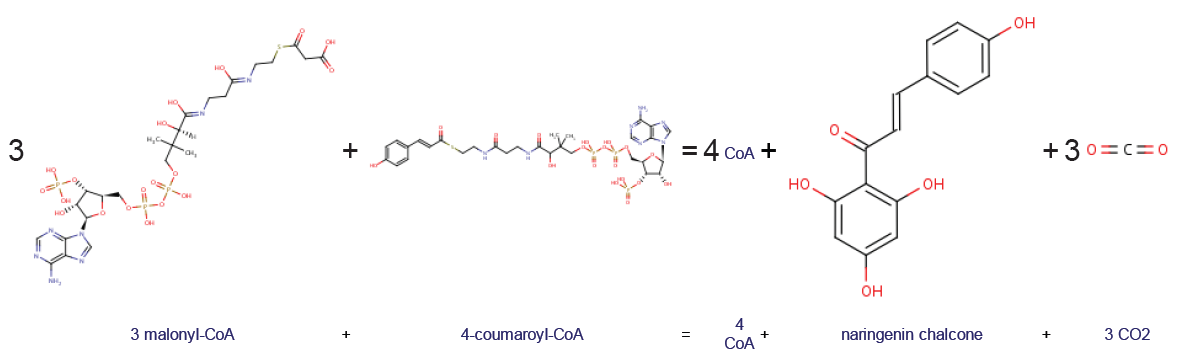
Naringenin chalcone is a common chalconoid (or chalcone, not to be confused with the compound chalcone). It is synthesized from 4-coumaroyl-CoA and malonyl-CoA by chalcone synthase (CHS), a key enzyme in the phenylpropanoid pathway. Naringenin chalcone can spontaneously cyclize to naringenin (a flavanone). In plant cells, this process is catalyzed by chalcone isomerase In enzymology, a chalcone isomerase () is an enzyme that catalyzes the chemical reaction :a chalcone \rightleftharpoons a flavanone Hence, this enzyme has one substrate, a chalcone, and one product, a flavanone. This enzyme belongs to the .... References Chalconoids {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chalconoid
Chalconoids Greek: χαλκός ''khalkós'', "copper", due to its color), also known as ''chalcones'', are natural phenols related to chalcone. They form the central core for a variety of important biological compounds. They show antibacterial, antifungal, antitumor and anti-inflammatory properties. Some chalconoids demonstrated the ability to block voltage-dependent potassium channels. Chalcones are also natural aromatase inhibitors. Chalcones are aromatic ketones with two phenyl rings that are also intermediates in the synthesis of many biological compounds. The closure of hydroxychalcones causes the formation of the flavonoid structure. Flavonoids are substances in the plant secondary metabolism with an array of biological activities. Chalconoids are also intermediates in the Auwers synthesis of flavones. Chemical properties Biosynthesis and metabolism Chalcone synthase is an enzyme responsible for the production of chalconoids in plants. Chalcone isomerase is responsi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chalcone
Chalcone is the organic compound C6H5C(O)CH=CHC6H5. It is an α,β-unsaturated ketone. A variety of important biological compounds are known collectively as chalcones or chalconoids. Chemical properties Chalcones have two absorption maxima at 280 nm and 340 nm. Synthesis Chalcone is usually prepared by an aldol condensation between benzaldehyde and acetophenone. : This reaction, which can be carried out without any solvent, is so reliable that it is used in as an example of green chemistry in undergraduate education. Biosynthesis Chalcones and chalconoids are synthesized in plants as secondary metabolites. The enzyme chalcone synthase, a type III polyketide synthase, is responsible for the biosynthesis of these compounds. The enzyme is found in all "higher" (vascular) and several "lower" ( non-vascular) plants. Potential pharmacology Chalcones and their derivatives demonstrate a wide range of biological activities including anti-inflammation. Some 2′-amino ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-coumaroyl-CoA
Coumaroyl-coenzyme A is the thioester of coenzyme-A and coumaric acid. Coumaroyl-coenzyme A is a central intermediate in the biosynthesis of myriad natural products found in plants. These products include Monolignol, lignols (precursors to lignin and lignocellulose), flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and other phenylpropanoids. Biosynthesis and significance It is generated in nature from phenylalanine, which is converted by Phenylalanine ammonia-lyase, PAL to trans-cinnamate. Trans-cinnamate is hydroxylated by trans-cinnamate 4-monooxygenase to give 4-hydroxycinnamate (i.e, coumarate). Coumarate is condensed with coenzyme-A in the presence of 4-coumarate-CoA ligase: :ATP + 4-coumarate + CoA \rightleftharpoons AMP + diphosphate + 4-coumaroyl-CoA. Enzymes using Coumaroyl-Coenzyme A * Anthocyanin 3-O-glucoside 6''-O-hydroxycinnamoyltransferase * Anthocyanin 5-aromatic acyltransferase * Chalcone synthase * 4-Coumarate-CoA ligase * 6'-Deoxychalcon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malonyl-CoA
Malonyl-CoA is a coenzyme A derivative of malonic acid. Functions It plays a key role in chain elongation in fatty acid biosynthesis and polyketide biosynthesis. Fatty acid biosynthesis Malonyl-CoA provides 2-carbon units to fatty acids and commits them to fatty acid chain synthesis. Malonyl-CoA is formed by carboxylating acetyl-CoA using the enzyme acetyl-CoA carboxylase. One molecule of acetyl-CoA joins with a molecule of bicarbonate,Nelson D, Cox M (2008) ''Lehninger principles of biochemistry''. 5th Ed: p. 806 requiring energy rendered from ATP. Malonyl-CoA is utilised in fatty acid biosynthesis by the enzyme malonyl coenzyme A:acyl carrier protein transacylase (MCAT). MCAT serves to transfer malonate from malonyl-CoA to the terminal thiol of ''holo''-acyl carrier protein (ACP). Polyketide biosynthesis MCAT is also involved in bacterial polyketide biosynthesis. The enzyme MCAT together with an acyl carrier protein (ACP), and a polyketide synthase (PKS) and chain-length f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chalcone Synthase
Chalcone synthase or naringenin-chalcone synthase (CHS) is an enzyme ubiquitous to higher plants and belongs to a family of polyketide synthase enzymes (PKS) known as type III PKS. Type III PKSs are associated with the production of chalcones, a class of organic compounds found mainly in plants as natural defense mechanisms and as synthetic intermediates. CHS was the first type III PKS to be discovered. It is the first committed enzyme in flavonoid biosynthesis. The enzyme catalyzes the conversion of 4-coumaroyl-CoA and malonyl-CoA to naringenin chalcone. Function CHS catalysis serves as the initial step for flavonoid biosynthesis. Flavonoids are important plant secondary metabolites that serve various functions in higher plants. These include pigmentation, UV protection, fertility, antifungal defense and the recruitment of nitrogen-fixing bacteria. CHS is believed to act as a central hub for the enzymes involved in the flavonoid pathway. Studies have shown that these enzyme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylpropanoid Pathway
The phenylpropanoids are a diverse family of organic compounds that are synthesized by plants from the amino acids phenylalanine and tyrosine. Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of coumaric acid, which is the central intermediate in phenylpropanoid biosynthesis. From 4-coumaroyl-CoA emanates the biosynthesis of myriad natural products including lignols (precursors to lignin and lignocellulose), flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids. The coumaroyl component is produced from cinnamic acid. Phenylpropanoids are found throughout the plant kingdom, where they serve as essential components of a number of structural polymers, provide protection from ultraviolet light, defend against herbivores and pathogens, and also mediate plant-pollinator interactions as floral pigments and scent compounds. Hydroxycinnamic acids Phenylalanine is first converted to cinnamic acid by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclization Reaction
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon (i.e., are carbocycles), none of the atoms are carbon (inorganic cyclic compounds), or where both carbon and non-carbon atoms are present (heterocyclic compounds). Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size (e.g., < 17 total atoms) numbers in the man ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naringenin
Naringenin is a flavorless, colorless flavanone, a type of flavonoid. It is the predominant flavanone in grapefruit, and is found in a variety of fruits and herbs. Structure Naringenin has the skeleton structure of a flavanone with three hydroxy groups at the 4', 5, and 7 carbons. It may be found both in the aglycol form, naringenin, or in its glycosidic form, naringin, which has the addition of the disaccharide neohesperidose attached via a glycosidic linkage at carbon 7. Like the majority of flavanones, naringenin has a single chiral center at carbon 2, although the optical purity is variable. Racemization of S(-)-naringenin has been shown to occur fairly quickly. Sources and bioavailability Naringenin and its glycoside has been found in a variety of herbs and fruits, including grapefruit, bergamot, sour orange, tart cherries, tomatoes, cocoa, Greek oregano, water mint, as well as in beans. Ratios of naringenin to naringin vary among sources, as do enantiomeric rati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavanone
The flavanones, a type of flavonoids, are various aromatic, colorless ketones derived from flavone that often occur in plants as glycosides. List of flavanones * Blumeatin * Butin * Eriodictyol * Hesperetin * Hesperidin * Homoeriodictyol * Isosakuranetin * Naringenin * Naringin * Pinocembrin * Poncirin * Sakuranetin * Sakuranin * Sterubin * Pinostrobin Metabolism The enzyme chalcone isomerase uses a chalcone-like compound to produce a flavanone. Flavanone 4-reductase is an enzyme that uses (2''S'')-flavan-4-ol The flavan-4-ols (3-deoxyflavonoids) are flavone-derived alcohols and a family of flavonoids. Flavan-4-ols are colorless precursor compounds that polymerize to form red phlobaphene pigments. They can be found in the sorghum. Glycosides (abacopteri ... and NADP+ to produce (2''S'')-flavanone, NADPH, and H+. Synthesis Numerous methods exist for the enantioselective chemical and biochemical synthesis of flavanones and related compounds. References External links ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chalcone Isomerase
In enzymology, a chalcone isomerase () is an enzyme that catalyzes the chemical reaction :a chalcone \rightleftharpoons a flavanone Hence, this enzyme has one substrate, a chalcone, and one product, a flavanone. This enzyme belongs to the family of isomerases, specifically the class of intramolecular lyases. The systematic name of this enzyme class is flavanone lyase (decyclizing). This enzyme is also called chalcone-flavanone isomerase. This enzyme participates in flavonoid biosynthesis. The ''Petunia hybrida'' (Petunia) genome contains two genes coding for very similar enzymes, ChiA and ChiB, but only the first seems to encode a functional chalcone isomerase. Structural studies As of late 2007, 7 structures have been solved for this class of enzymes, with PDB accession codes , , , , , , and . Chalcone isomerase has a core 2-layer alpha/beta structure A structure is an arrangement and organization of interrelated elements in a material object or system, or the object ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |