|
Monoterpenoids
Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C10H16. Monoterpenes may be linear (acyclic) or contain rings (monocyclic and bicyclic). Modified terpenes, such as those containing oxygen functionality or missing a methyl group, are called monoterpenoids. Monoterpenes and monoterpenoids are diverse. They have relevance to the pharmaceutical, cosmetic, agricultural, and food industries. Biosynthesis Monoterpenes are derived biosynthetically from units of isopentenyl pyrophosphate, which is formed from acetyl-CoA via the intermediacy of mevalonic acid in the HMG-CoA reductase pathway. An alternative, unrelated biosynthesis pathway of IPP is known in some bacterial groups and the plastids of plants, the so-called MEP-(2-methyl-D-erythritol-4-phosphate) pathway, which is initiated from C5 sugars. In both pathways, IPP is isomerized to DMAPP by the enzyme isopentenyl pyrophosphate isomerase. Geranyl pyrophosphate is the precursor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Terpene
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n > 1. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes are further classified by the number of carbons: monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), as examples. The terpene alpha-pinene, is a major component of the common solvent, turpentine. History and terminology The term ''terpene'' was coined in 1866 by the German chemist August Kekulé to denote all hydrocarbons having the empirical formula C10H16, of which camphene was one. Previously, many hydrocarbons having the empirical formula C10H16 had been called "camphene", but many other hydrocarbons of the same composition had had different names. Kekulé coined the term "terpene" in order to reduce the confusion. The name "terpene" is a shortened form of "terpentine", an obsolete spelling of "turpentine". Although sometimes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borneol
Borneol is a bicyclic organic compound and a terpene derivative. The hydroxyl group in this compound is placed in an '' endo'' position. The exo diastereomer is called isoborneol. Being chiral, borneol exists as enantiomers, both of which are found in nature. Reactions Borneol is oxidized to the ketone ( camphor). Occurrence The compound was named in 1842 by the French chemist Charles Frédéric Gerhardt. Borneol can be found in several species of ''Heterotheca'', ''Artemisia'', ''Rosmarinus officinalis'' (rosemary) ''Dipterocarpaceae'', ''Blumea balsamifera'' and '' Kaempferia galanga''. It is one of the chemical compounds found in castoreum. This compound is gathered from the beaver's plant food. Synthesis Borneol can be synthesized by reduction of camphor by the Meerwein–Ponndorf–Verley reduction (a reversible process). Reduction of camphor with sodium borohydride (fast and irreversible) gives instead the diastereomer isoborneol. : Uses Whereas ''d''-borneol w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Camphor
Camphor () is a waxy, colorless solid with a strong aroma. It is classified as a terpenoid and a cyclic ketone. It is found in the wood of the camphor laurel ('' Cinnamomum camphora''), a large evergreen tree found in East Asia; and in the kapur tree ( ''Dryobalanops'' sp.), a tall timber tree from South East Asia. It also occurs in some other related trees in the laurel family, notably '' Ocotea usambarensis''. Rosemary leaves (''Rosmarinus officinalis'') contain 0.05 to 0.5% camphor, while camphorweed (''Heterotheca'') contains some 5%. A major source of camphor in Asia is camphor basil (the parent of African blue basil). Camphor can also be synthetically produced from oil of turpentine. The compound is chiral, existing in two possible enantiomers as shown in the structural diagrams. The structure on the left is the naturally occurring (+)-camphor ((1''R'',4''R'')-bornan-2-one), while its mirror image shown on the right is the (−)-camphor ((1''S'',4''S'')-bornan-2-one). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thujene
Thujene (or α-thujene) is a natural organic compound classified as a monoterpene. It is found in the essential oils of a variety of plants, and contributes pungency to the flavor of some herbs such as Summer savory.''PDR for Herbal Medicines'', Third Edition, Joerg Gruenwald (Editor), page 802. The term ''thujene'' usually refers to α-thujene. A less common chemically related double-bond isomer is known as β-thujene (or 2-thujene). Another double-bond isomer is known as sabinene. {, class="toccolours" border="1" style="margin: 0 0 1em 1em; border-collapse: collapse;" , colspan="3" align="center" , Chemical structure comparison , - , , , , - , align="center", α-Thujene , align="center", β-Thujene , align="center", Sabinene See also * Thujone * Umbellulone Umbellulone is a headache-inducing monoterpene ketone found in the leaves of the tree ''Umbellularia californica'', sometimes known as the "headache tree". It is hypothesized to cause headaches by influenci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Camphene
Camphene is a bicyclic organic compound. It is one of the most pervasive monoterpenes. As for other terpenes, it is insoluble in water, flammable, colorless, and has a pungent smell. It is a minor constituent of many essential oils such as turpentine, cypress oil, camphor oil, citronella oil, neroli, ginger oil, valerian, and mango. It is produced industrially by isomerization of the more common alpha-pinene using a solid acid catalyst such as titanium dioxide. Camphene is used in the preparation of fragrances and as a food additive for flavoring. These include isobornyl acetate. Biosynthesis Camphene is biosynthesized from linalyl pyrophosphate via a sequence of carbocation A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encountere ...ic intermediates. References {{Authority con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
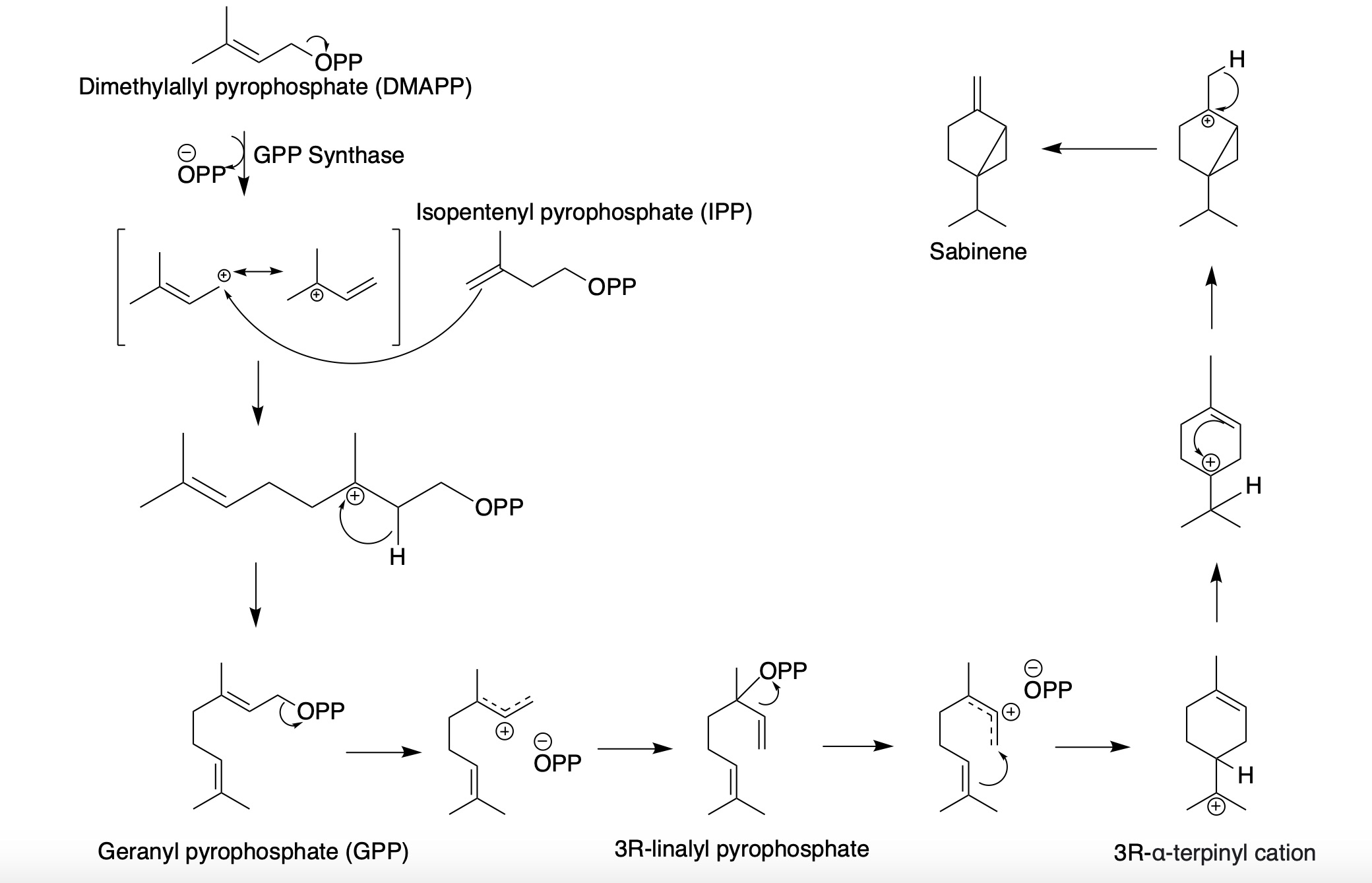
Sabinene
Sabinene is a natural bicyclic monoterpene with the molecular formula C10H16. It is isolated from the essential oils of a variety of plants including Marjoram, holm oak (''Quercus ilex'') and Norway spruce (''Picea abies''). It has a strained ring system with a cyclopentane ring fused to a cyclopropane ring. Sabinene is one of the chemical compounds that contributes to the spiciness of black pepper and is a major constituent of carrot seed oil. It also occurs in tea tree oil at a low concentration. It is also present in the essential oil obtained from nutmeg, ''Laurus nobilis'', and ''Clausena anisata''. Biosynthesis Sabinene, a bicyclic monoterpene, is present in the (+) and (-) enantiomers. It is biosynthesized from the common terpenoid precursor, geranyl pyrophosphate (GPP) that undergoes polycyclization catalyzed by sabinene synthase (SabS). GPP is formed from the terpenoid synthesis pathway with the starter units, isopentenyl pyrophosphate (IPP) and dimethylallyl py ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carene
3-Carene is a bicyclic monoterpene consisting of fused cyclohexene and cyclopropane rings. It occurs as a constituent of turpentine, with a content as high as 42% depending on the source. Carene has a sweet and pungent odor, best described as a combination of fir needles, musky earth, and damp woodlands. A colorless liquid, it is not soluble in water, but miscible with fats and oils. It is chiral, occurring naturally both as the racemate and enantio-enriched forms. Reactions and uses Treatment with peracetic acid gives 3,4-caranediol. Pyrolysis over ferric oxide induces rearrangement, giving ''p''-cymene. Carene is used in the perfume industry and as a chemical intermediate. Because carene can be found in cannabis naturally, it can also be found in cannabis distillates. Greater concentrations of carene in a distillate give it an earthier taste and smell. 3-Carene is also present in mango, giving the fruit a characteristic pine A pine is any conifer tree or shrub in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linalool
Linalool () refers to two enantiomers of a naturally occurring terpene alcohol found in many flowers and spice plants. Linalool has multiple commercial applications, the majority of which are based on its pleasant scent (floral, with a touch of spiciness). A colorless oil, linalool is classified as an acyclic monoterpenoid. In plants, it is a metabolite, a volatile oil component, an antimicrobial agent, and an aroma compound. Linalool has uses in manufacturing of soaps, fragrances, food additives as flavors, household products, and insecticides. Esters of linalool are referred to as linalyl, e.g. linalyl pyrophosphate, an isomer of geranyl pyrophosphate. The word ''linalool'' is based on '' linaloe'' (a type of wood) and the suffix '. In food manufacturing, it may be called ''coriandrol''. Occurrence Both enantiomeric forms are found in nature: (''S'')-linalool is found, for example, as a major constituent of the essential oils of coriander (''Coriandrum sativum'' L.), cymbopo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tropolone
Tropolone is an organic compound with the chemical formula . It is a pale yellow solid that is soluble in organic solvents. The compound has been of interest to research chemists because of its unusual electronic structure and its role as a ligand precursor. Although not usually prepared from tropone, it can be viewed as its derivative with a hydroxyl group in the 2-position. Synthesis and reactions Many methods have been described for the synthesis of tropolone. One involves bromination of 1,2-cycloheptanedione with ''N''-bromosuccinimide followed by dehydrohalogenation at elevated temperatures, while another uses acyloin condensation of the ethyl ester of pimelic acid the acyloin again followed by oxidation by bromine. : An alternate route is a +2cycloaddition of cyclopentadiene with a ketene to give a bicyclo .2.0eptyl structure, followed by hydrolysis and breakage of the fusion bond to give the single ring: : Thy hydroxyl group of tropolone is acidic, having a p''K''a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hinokitiol
Hinokitiol (β-thujaplicin) is a natural monoterpenoid found in the wood of trees in the family Cupressaceae. It is a tropolone derivative and one of the thujaplicins. Hinokitiol is used in oral and skin care products, and is a food additive used in Japan. History Hinokitiol was discovered by a Japanese chemist Tetsuo Nozoe in 1936. It was isolated from the essential oil component of the heartwood of '' Taiwanese hinoki'', from which the compound ultimately adopted its name. Hinokitiol is the first non-benzenoid aromatic compound identified. The compound has a heptagonal molecular structure and was first synthesyzed by Ralph Raphael in 1951. Due to its iron-chelating activity, hinokitiol has been called an "Iron Man molecule" in the scientific media, which is ironic because Tetsuo is translated into English as "Iron Man". Taiwanese hinoki is native to East Asian countries, particularly to Japan and Taiwan. Hinokitiol has also been found in other trees of the ''Cupressaceae'' fa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carvone
Carvone is a member of a family of chemicals called terpenoids. Carvone is found naturally in many essential oils, but is most abundant in the oils from seeds of caraway (''Carum carvi''), spearmint (''Mentha spicata''), and dill. Uses Both carvones are used in the food and flavor industry. ''R''-(−)-Carvone is also used for air freshening products and, like many essential oils, oils containing carvones are used in aromatherapy and alternative medicine. ''S''-(+)-Carvone has shown a suppressant effect against high-fat diet induced weight gain in mice. Food applications As the compound most responsible for the flavor of caraway, dill and spearmint, carvone has been used for millennia in food. Wrigley's Spearmint Gum and spearmint flavored Life Savers are major users of natural spearmint oil from ''Mentha spicata''. Caraway seed is extracted with alcohol to make the European drink Kümmel. Agriculture ''S''-(+)-Carvone is also used to prevent premature sprouting of potatoes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |