|
Miller–Urey Experiment
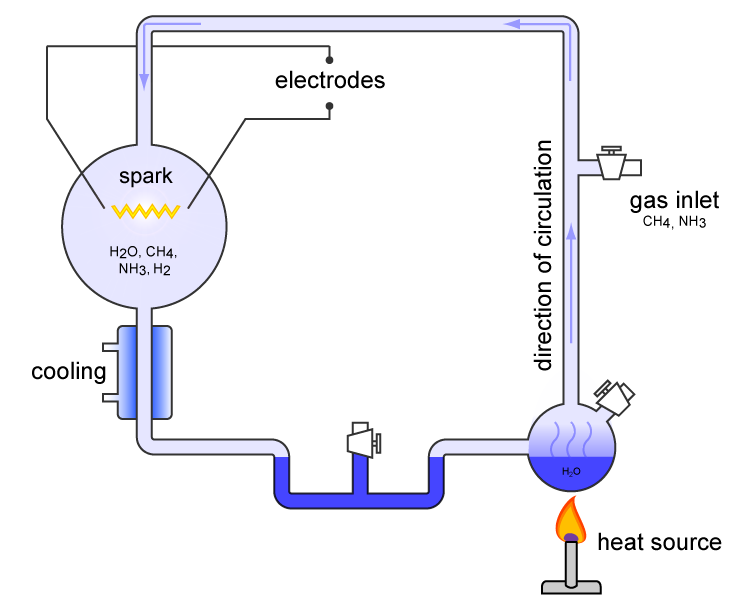
The Miller–Urey experiment (or Miller experiment) is a famous chemistry experiment that simulated the conditions thought at the time (1952) to be present in the atmosphere of the early, prebiotic Earth, in order to test the hypothesis of the chemical origin of life under those conditions. The experiment used water (H2O), methane (CH4), ammonia (NH3), hydrogen (H2), and an electric arc (the latter simulating hypothesized lightning). At the time, it supported Alexander Oparin's and J. B. S. Haldane's hypothesis that the hypothesized conditions on the primitive Earth favored chemical reactions that synthesized more complex organic compounds from simpler inorganic precursors. One of the most famous experiments of all time, it is considered to be groundbreaking, and to be the classic experiment investigating abiogenesis. It was performed in 1952 by Stanley Miller, supervised by Harold Urey at the University of Chicago, and published the following year. After Miller's death in 20 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Earth makes it an economically attractive fuel, although capturing and storing it poses technical challenges due to its gaseous state under normal conditions for temperature and pressure. Naturally occurring methane is found both below ground and under the seafloor and is formed by both geological and biological processes. The largest reservoir of methane is under the seafloor in the form of methane clathrates. When methane reaches the surface and the atmosphere, it is known as atmospheric methane. The Earth's atmospheric methane concentration has increased by about 150% since 1750, and it accounts for 20% of the total radiative forcing from all of the long-lived and globally mixed greenhouse gases. It has also been detected on other plane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of California, San Diego
The University of California, San Diego (UC San Diego or colloquially, UCSD) is a public university, public Land-grant university, land-grant research university in San Diego, California. Established in 1960 near the pre-existing Scripps Institution of Oceanography, UC San Diego is the southernmost of the ten campuses of the University of California, and offers over 200 undergraduate and graduate degree programs, enrolling 33,096 undergraduate and 9,872 graduate students. The university occupies near the coast of the Pacific Ocean, with the main campus resting on approximately . UC San Diego is ranked among the best universities in the world by major college and university rankings. UC San Diego consists of twelve undergraduate, graduate and professional schools as well as seven undergraduate residential colleges. It received over 140,000 applications for undergraduate admissions in Fall 2021, making it the second most applied-to university in the United States. UC San Diego H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jeffrey Bada
Jeffrey L. Bada (born September 10, 1942) is an American chemist known for his works in the study of the origin of life. He is Distinguished Research Professor of Marine Chemistry and former Director of the NASA Specialized Center of Research and Training (NSCORT) in Exobiology at the Scripps Institution of Oceanography, University of California, San Diego. Bada has played a pioneering role in the development of the Mars Organic Detector (MOD) instrument package that is designed to search for amino acids and other organic compounds directly on the surface of Mars during future ESA and NASA missions. Education and career Bada studied at the San Diego State University and obtained BS in chemistry in 1965. He wanted to become a theoretical chemist, applying quantum mechanics to chemistry and had no prior interest in prebiotic chemistry. Then he met Stanley Miller at the University of California, San Diego (UCSD) who inspired him to take up the spark discharge experiment a step for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspartic Acid
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. Like all other amino acids, it contains an amino group and a carboxylic acid. Its α-amino group is in the protonated –NH form under physiological conditions, while its α-carboxylic acid group is deprotonated −COO− under physiological conditions. Aspartic acid has an acidic side chain (CH2COOH) which reacts with other amino acids, enzymes and proteins in the body. Under physiological conditions (pH 7.4) in proteins the side chain usually occurs as the negatively charged aspartate form, −COO−. It is a non-essential amino acid in humans, meaning the body can synthesize it as needed. It is encoded by the codons GAU and GAC. D-Aspartate is one of two D-amino acids commonly found in mammals. .html" ;"title="/sup>">/sup> In proteins aspartate sidechains are often hydrogen bonded to form asx turns or asx motifs, which frequently occur at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alanine
Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group side chain. Consequently, its IUPAC systematic name is 2-aminopropanoic acid, and it is classified as a nonpolar, aliphatic α-amino acid. Under biological conditions, it exists in its zwitterionic form with its amine group protonated (as −NH3+) and its carboxyl group deprotonated (as −CO2−). It is non-essential to humans as it can be synthesised metabolically and does not need to be present in the diet. It is encoded by all codons starting with GC (GCU, GCC, GCA, and GCG). The L-isomer of alanine (left-handed) is the one that is incorporated into proteins. L-alanine is second only to leucine in rate of occurrence, accounting for 7.8% of the primary structure in a sample of 1,150 proteins. The right-handed form, D-alanine, occurs in p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid (carbamic acid is unstable), with the chemical formula NH2‐ CH2‐ COOH. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (GGU, GGC, GGA, GGG). Glycine is integral to the formation of alpha-helices in secondary protein structure due to its compact form. For the same reason, it is the most abundant amino acid in collagen triple-helices. Glycine is also an inhibitory neurotransmitter – interference with its release within the spinal cord (such as during a ''Clostridium tetani'' infection) can cause spastic paralysis due to uninhibited muscle contraction. It is the only achiral proteinogenic amino acid. It can fit into hydrophilic or hydrophobic environments, due to its minimal side chain of only one hydrogen atom. History and etymology Glycine was discovered in 1820 by the French chemist He ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paper Chromatography
Paper chromatography is an analytical method used to separate coloured chemicals or substances. It is now primarily used as a teaching tool, having been replaced in the laboratory by other chromatography methods such as thin-layer chromatography (TLC). A paper chromatography variant, two-dimensional chromatography, involves using two solvents and rotating the paper 90° in between. This is useful for separating complex mixtures of compounds having similar polarity, for example, amino acids. The setup has three components. The mobile phase is a solution that travels up the stationary phase, due to capillary action. The mobile phase is generally a mixture of non-polar organic solvent, while the stationary phase is polar inorganic solvent water. Here paper is used to support the stationary phase, water. Polar water molecules are held inside the void space of the cellulose network of the host paper. The difference between TLC and paper chromatography is that the stationary phase in TL ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lightning
Lightning is a naturally occurring electrostatic discharge during which two electric charge, electrically charged regions, both in the atmosphere or with one on the land, ground, temporarily neutralize themselves, causing the instantaneous release of an average of one Joule, gigajoule of energy. This discharge may produce a wide range of electromagnetic radiation, from heat created by the rapid movement of electrons, to brilliant flashes of visible light in the form of black-body radiation. Lightning causes thunder, a sound from the shock wave which develops as gases in the vicinity of the discharge experience a sudden increase in pressure. Lightning occurs commonly during thunderstorms as well as other types of energetic weather systems, but volcanic lightning can also occur during volcanic eruptions. The three main kinds of lightning are distinguished by where they occur: either inside a single Cumulonimbus cloud, thundercloud (intra-cloud), between two clouds (cloud-to-cl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Evaporation
Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase. High concentration of the evaporating substance in the surrounding gas significantly slows down evaporation, such as when humidity affects rate of evaporation of water. When the molecules of the liquid collide, they transfer energy to each other based on how they collide. When a molecule near the surface absorbs enough energy to overcome the vapor pressure, it will escape and enter the surrounding air as a gas. When evaporation occurs, the energy removed from the vaporized liquid will reduce the temperature of the liquid, resulting in evaporative cooling. On average, only a fraction of the molecules in a liquid have enough heat energy to escape from the liquid. The evaporation will continue until an equilibrium is reached when the evaporation of the liquid is equal to its condensation. In an enclosed environment, a liquid will evaporate until the surrounding air is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |