|
Mathias Uhlén
Mathias Uhlén (born May 1954) is a Swedish scientist and Professor of Microbiology at Royal Institute of Technology (KTH), Stockholm. After a post-doc period at the EMBL in Heidelberg, Germany, he became professor in microbiology at KTH in 1988. His research is focused on protein science, antibody engineering and precision medicine and range from basic research in human and microbial biology to more applied research, including clinical applications. He is member of several academies and societies, including Royal Swedish Academy of Science (KVA), National Academy of Engineering (NAE) and the Swedish Academy of Engineering Science (IVA). Dr Uhlen was the Founding Director of the national infrastructure Science for Life Laboratory ( SciLifeLab) from 2010 to 2015. Research His group was the first to describe a number of innovations in science including: Protein engineering This broad concept of Affinity-based protein engineering was developed to use specific binding (affin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Engineering
Protein engineering is the process of developing useful or valuable proteins through the design and production of unnatural polypeptides, often by altering amino acid sequences found in nature. It is a young discipline, with much research taking place into the understanding of protein folding and recognition for protein design principles. It has been used to improve the function of many enzymes for industrial catalysis. It is also a product and services market, with an estimated value of $168 billion by 2017. There are two general strategies for protein engineering: rational protein design and directed evolution. These methods are not mutually exclusive; researchers will often apply both. In the future, more detailed knowledge of protein structure and function, and advances in high-throughput screening, may greatly expand the abilities of protein engineering. Eventually, even unnatural amino acids may be included, via newer methods, such as expanded genetic code, that allow e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleotide
Nucleotides are Organic compound, organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules within all Life, life-forms on Earth. Nucleotides are obtained in the diet and are also synthesized from common Nutrient, nutrients by the liver. Nucleotides are composed of three subunit molecules: a nucleobase, a pentose, five-carbon sugar (ribose or deoxyribose), and a phosphate group consisting of one to three phosphates. The four nucleobases in DNA are guanine, adenine, cytosine, and thymine; in RNA, uracil is used in place of thymine. Nucleotides also play a central role in metabolism at a fundamental, cellular level. They provide chemical energy—in the form of the nucleoside triphosphates, adenosine triphosphate (ATP), guanosine triphosphate (GTP), cytidine triphosphate (CTP), and uridine triph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid Phase Sequencing
The principle of solid phase DNA sequencing was described in 1989 based on binding of biotinylated DNA to streptavidin-coated magnetic beads and elution of single DNA strands selectively using alkali. The method allowed robotic applications suitable for clinical sequencing, but the magnetic handling has also found frequent use in many molecular applications, including sample handling for DNA diagnostics. The use of solid phase methods for DNA handling is now frequently used as an integrated part of many of the next generation DNA sequencing methods, as well as numerous molecular diagnostics Molecular diagnostics is a collection of techniques used to analyze biological markers in the genome and proteome, and how their cells express their genes as proteins, applying molecular biology to medical tests, medical testing. In medicine th ... applications. References {{reflist Biotechnology ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
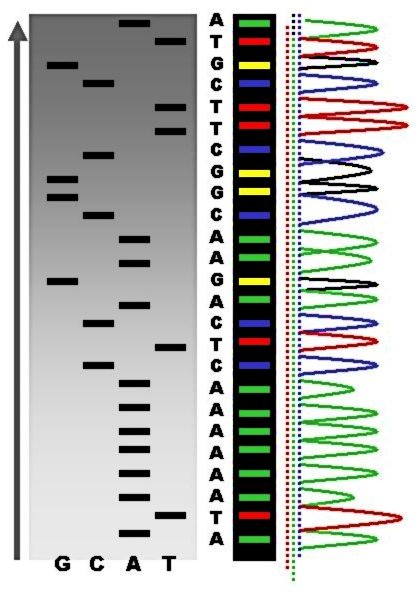
DNA Sequencing
DNA sequencing is the process of determining the nucleic acid sequence – the order of nucleotides in DNA. It includes any method or technology that is used to determine the order of the four bases: adenine, thymine, cytosine, and guanine. The advent of rapid DNA sequencing methods has greatly accelerated biological and medical research and discovery. Knowledge of DNA sequences has become indispensable for basic biological research, Genographic Project, DNA Genographic Projects and in numerous applied fields such as medical diagnosis, biotechnology, forensic biology, virology and biological systematics. Comparing healthy and mutated DNA sequences can diagnose different diseases including various cancers, characterize antibody repertoire, and can be used to guide patient treatment. Having a quick way to sequence DNA allows for faster and more individualized medical care to be administered, and for more organisms to be identified and cataloged. The rapid advancements in DNA seque ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DNA Polymerase
A DNA polymerase is a member of a family of enzymes that catalyze the synthesis of DNA molecules from nucleoside triphosphates, the molecular precursors of DNA. These enzymes are essential for DNA replication and usually work in groups to create two identical DNA duplexes from a single original DNA duplex. During this process, DNA polymerase "reads" the existing DNA strands to create two new strands that match the existing ones. These enzymes catalysis, catalyze the chemical reaction : deoxynucleoside triphosphate + DNAn pyrophosphate + DNAn+1. DNA polymerase adds nucleotides to the Directionality (molecular biology), three prime (3')-end of a DNA strand, one nucleotide at a time. Every time a Cell division, cell divides, DNA polymerases are required to duplicate the cell's DNA, so that a copy of the original DNA molecule can be passed to each daughter cell. In this way, genetic information is passed down from generation to generation. Before replication can take place, an enzy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Therapeutic Antibodies
Monoclonal antibodies (mAbs) have varied therapeutic uses. It is possible to create a mAb that binds specifically to almost any extracellular target, such as cell surface proteins and cytokines. They can be used to render their target ineffective (e.g. by preventing receptor binding), to induce a specific cell signal (by activating receptors), to cause the immune system to attack specific cells, or to bring a drug to a specific cell type (such as with radioimmunotherapy which delivers cytotoxic radiation). Major applications include cancer, autoimmune diseases, asthma, organ transplants, blood clot prevention, and certain infections. Antibody structure and function Immunoglobulin G ( IgG) antibodies are large heterodimeric molecules, approximately 150 kDa and are composed of two kinds of polypeptide chain, called the heavy (~50kDa) and the light chain (~25kDa). The two types of light chains are kappa (κ) and lambda (λ). By cleavage with enzyme papain, the Fab (''fragment-an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Streptavidin
Streptavidin is a 52 Atomic mass unit, kDa protein (tetramer) purified from the bacterium ''Streptomyces avidinii''. Streptavidin Homotetramer, homo-tetramers have an extraordinarily high affinity for biotin (also known as vitamin B7 or vitamin H). With a dissociation constant (Kd) on the order of ≈10−14 mol/L, the binding of biotin to streptavidin is one of the strongest non-covalent interactions known in nature. Streptavidin is used extensively in molecular biology and bionanotechnology due to the streptavidin-biotin complex's resistance to organic solvents, denaturants (e.g. guanidinium chloride), detergents (e.g. Sodium dodecyl sulfate, SDS, Triton X-100), proteolytic enzymes, and extremes of temperature and pH. Structure The crystal structure of streptavidin with biotin bound was reported by two groups in 1989. The structure was solved using multi wavelength anomalous diffraction by Hendrickson et al. at Columbia University and using multiple isomorphous replacemen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biotin
Biotin (also known as vitamin B7 or vitamin H) is one of the B vitamins. It is involved in a wide range of metabolic processes, both in humans and in other organisms, primarily related to the utilization of fats, carbohydrates, and amino acids. The name ''biotin'', borrowed from the German , derives from the Ancient Greek word (; 'life') and the suffix "-in" (a suffix used in chemistry usually to indicate 'forming'). Biotin appears as a white, needle-like crystalline solid. Chemical description Biotin is classified as a heterocyclic compound, with a sulfur-containing tetrahydrothiophene ring fused to a ureido group. A C5-carboxylic acid side chain is appended to the former ring. The ureido ring, containing the −N−CO−N− group, serves as the carbon dioxide carrier in carboxylation reactions. Biotin is a coenzyme for five carboxylase enzymes, which are involved in the catabolism of amino acids and fatty acids, synthesis of fatty acids, and gluconeogenesis. Biotinylat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
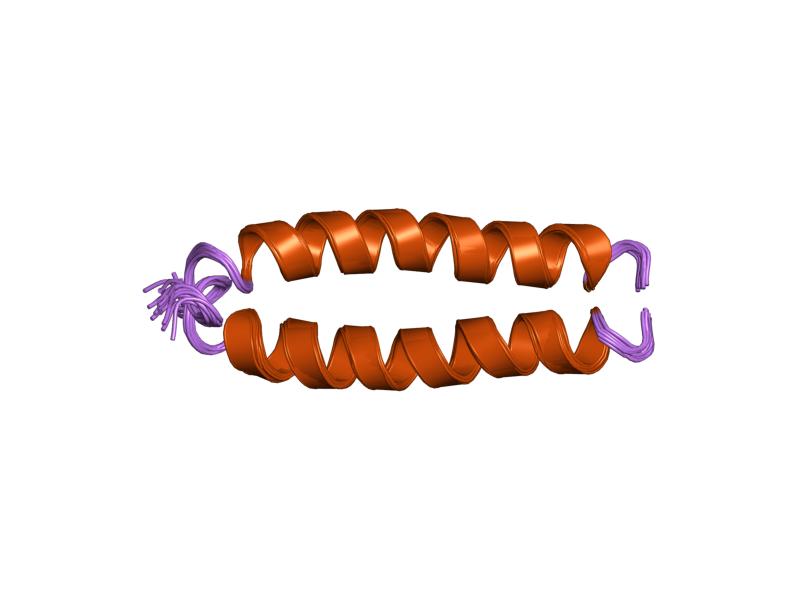
Affibody Molecule
Affibody molecules are small, robust proteins engineered to bind to a large number of target proteins or peptides with high affinity, imitating monoclonal antibodies, and are therefore a member of the family of antibody mimetics. Affibody molecules are used in biochemical research and are being developed as potential new biopharmaceutical drugs. These molecules can be used for molecular recognition in diagnostic and therapeutic applications. Development As with other antibody mimetics, the idea behind developing the Affibody molecule was to apply a combinatorial protein engineering approach on a small and robust protein scaffold. The aim was to generate new binders capable of specific binding to different target proteins with almost good affinity, while retaining the favorable folding and stability properties, and ease of bacterial expression of the parent molecule. The original Affibody protein scaffold was designed based on the Z domain (the immunoglobulin G binding domain) of p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fusion Protein
Fusion proteins or chimeric (kī-ˈmir-ik) proteins (literally, made of parts from different sources) are proteins created through the joining of two or more genes that originally coded for separate proteins. Translation of this '' fusion gene'' results in a single or multiple polypeptides with functional properties derived from each of the original proteins. ''Recombinant fusion proteins'' are created artificially by recombinant DNA technology for use in biological research or therapeutics. '' Chimeric'' or ''chimera'' usually designate hybrid proteins made of polypeptides having different functions or physico-chemical patterns. ''Chimeric mutant proteins'' occur naturally when a complex mutation, such as a chromosomal translocation, tandem duplication, or retrotransposition creates a novel coding sequence containing parts of the coding sequences from two different genes. Naturally occurring fusion proteins are commonly found in cancer cells, where they may function as oncop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Tag
Protein tags are peptide sequences genetically grafted onto a recombinant protein. Tags are attached to proteins for various purposes. They can be added to either end of the target protein, so they are either C-terminus or N-terminus specific or are both C-terminus and N-terminus specific. Some tags are also inserted at sites within the protein of interest; they are known as internal tags. Affinity tags are appended to proteins so that they can be purified from their crude biological source using an affinity technique. Affinity tags include chitin binding protein (CBP), maltose binding protein (MBP), Strep-tag and glutathione-S-transferase (GST). The Polyhistidine-tag, poly(His) tag is a widely used protein tag, which binds to matrices bearing immobilized metal ions. Solubilization tags are used, especially for recombinant proteins expressed in species such as ''E. coli'', to assist in the proper folding in proteins and keep them from aggregating in inclusion bodies. These tags ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |