|
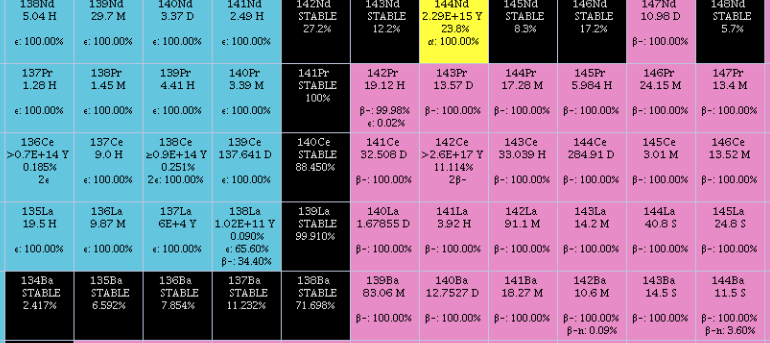
Lanthanum Oxyfluoride
Lanthanum(III) oxyfluoride is an inorganic compound of lanthanum, fluorine, and oxygen with the chemical formula LaOF. Synthesis Several methods of synthesizing LaOF are known: * Hydrolysis of lanthanum fluoride by superheated steam: :: * Decomposition of lanthanum fluoride semihydrate when heated in vacuum: :: * Reacting lanthanum oxide with lanthanum fluoride in vacuum A vacuum is a space devoid of matter. The word is derived from the Latin adjective ''vacuus'' for "vacant" or "void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressure. Physicists often dis ...: :: Physical properties The compound forms colorless crystals. Uses The compound is used to produce thin films. References {{Fluorine compounds Lanthanum compounds Oxyfluorides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yttrium Oxyfluoride
Yttrium oxyfluoride is an inorganic chemical compound with the formula . Under normal conditions, the compound is a colorless solid. Synthesis Yttrium oxyfluoride may be formed in the decomposition of crystalline hydrate of yttrium fluoride upon heating to 900 °C in a vacuum: :: \mathsf Yttrium oxyfluoride can also be made by hydrolysis of yttrium fluoride with superheated steam at 800 °C: :: \mathsf Physical properties Yttrium oxyfluoride forms colorless crystals of tetragonal crystal system In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square ...; its cell parameters are: a = 0.3910 nm, c = 0.5431 nm. According to hexagonal crystal family, the cell parameters are: a = 0.38727 nm, c = 1.897 nm, Z = 6. Applications Stable yttrium oxyfluoride material ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thorium Oxyfluoride
Thorium oxyfluoride is an inorganic compound of thorium metal, fluorine, and oxygen with the chemical formula . Synthesis *Thorium oxyfluoride can be prepared from partial hydrolysis of thorium tetrafluoride in moist air at elevated temperatures, about 1000 °C. ::ThF4 + H2O -> ThOF2 + 2HF * Reaction of thorium tetrafluoride with thorium dioxide Thorium dioxide (ThO2), also called thorium(IV) oxide, is a crystalline solid, often white or yellow in colour. Also known as thoria, it is produced mainly as a by-product of lanthanide and uranium production. Thorianite is the name of the minera ... at 600 °C: ::ThF4 + ThO2 -> 2ThOF2 Physical properties The compound forms white, insoluble amorphous powder. Uses The compound is used as a protective coating on reflective surfaces. References {{Fluorine compounds Thorium compounds Oxyfluorides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. Some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, etc.), carbon monoxide, carbon dioxide, carbides, and the following salts of inorganic anions: carbonates, cyanides, cyanates, and thiocyanates. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it does not occur within living things. History Friedrich Wöhler's conversion of ammonium cyanate into urea in 1828 is often cited as the starting point of modern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between lanthanum and lutetium in the periodic table, of which lanthanum is the first and the prototype. Lanthanum is traditionally counted among the rare earth elements. Like most other rare earth elements, the usual oxidation state is +3. Lanthanum has no biological role in humans but is essential to some bacteria. It is not particularly toxic to humans but does show some antimicrobial activity. Lanthanum usually occurs together with cerium and the other rare earth elements. Lanthanum was first found by the Swedish chemist Carl Gustaf Mosander in 1839 as an impurity in cerium nitrate – hence the name ''lanthanum'', from the Ancient Greek (), meaning 'to lie hidden'. Although it is classified as a rare earth element, lanthanum is the 28th most abund ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element with the symbol F and atomic number 9. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. As the most electronegative reactive element, it is extremely reactive, as it reacts with all other elements except for the light inert gases. Among the elements, fluorine ranks 24th in universal abundance and 13th in terrestrial abundance. Fluorite, the primary mineral source of fluorine which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning 'flow' gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist Henri Moissan isolate elemental fluorine using low-temperature electrolysis, a process still employed for modern pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and fats, as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the substance and w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanum(III) Fluoride
Lanthanum trifluoride is a refractory ionic compound of lanthanum and fluorine. The LaF3 structure Bonding is ionic with lanthanum highly coordinated. The cation sits at the center of a trigonal prism. Nine fluorine atoms are close: three at the bottom corners of the trigonal prism, three in the faces of the trigonal prism, and three at top corners of the trigonal prism. There are also two fluorides a little further away above and below the prism. The cation can be considered 9-coordinate or 11-coordinate. At 300 K, the structure allows the formation of Schottky defects with an activation energy of 0.07 eV, and free flow of fluoride ions with an activation energy of 0.45 eV, making the crystal unusually electrically conductive. The larger sized rare earth elements ( lanthanides), which are those with smaller atomic number, also form trifluorides with the LaF3 structure. Some actinides do as well. Applications This white salt is sometimes used as the "high-i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superheated Steam
Superheated steam is steam at a temperature higher than its vaporization point at the absolute pressure where the temperature is measured. Superheated steam can therefore cool (lose internal energy) by some amount, resulting in a lowering of its temperature without changing state (i.e., condensing) from a gas, to a mixture of saturated vapor and liquid. If unsaturated steam (a mixture which contains both water vapor and liquid water droplets) is heated at constant pressure, its temperature will also remain constant as the vapor quality (think dryness, or percent saturated vapor) increases towards 100%, and becomes dry (i.e., no saturated liquid) saturated steam. Continued heat input will then "super" heat the dry saturated steam. This will occur if saturated steam contacts a surface with a higher temperature. Superheated steam and liquid water cannot coexist under thermodynamic equilibrium, as any additional heat simply evaporates more water and the steam will become saturated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vacuum
A vacuum is a space devoid of matter. The word is derived from the Latin adjective ''vacuus'' for "vacant" or "void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressure. Physicists often discuss ideal test results that would occur in a ''perfect'' vacuum, which they sometimes simply call "vacuum" or free space, and use the term partial vacuum to refer to an actual imperfect vacuum as one might have in a laboratory or in space. In engineering and applied physics on the other hand, vacuum refers to any space in which the pressure is considerably lower than atmospheric pressure. The Latin term ''in vacuo'' is used to describe an object that is surrounded by a vacuum. The ''quality'' of a partial vacuum refers to how closely it approaches a perfect vacuum. Other things equal, lower gas pressure means higher-quality vacuum. For example, a typical vacuum cleaner produces enough suction to reduce air pressure by around 20%. But hig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanum(III) Oxide
Lanthanum(III) oxide, also known as lanthana, chemical formula , is an inorganic compound containing the rare earth element lanthanum and oxygen. It is used in some ferroelectric materials, as a component of optical materials, and is a feedstock for certain catalysts, among other uses. Properties Lanthanum oxide is a white solid that is insoluble in water, but dissolves in acidic solutions. absorbs moisture from air, converts to lanthanum hydroxide. Lanthanum oxide has p-type semiconducting properties and a band gap of approximately 5.8 eV. Its average room temperature resistivity is 10 kΩ·cm, which decreases with an increase in temperature. has the lowest lattice energy of the rare earth oxides, with very high dielectric constant, ε = 27. Structure At low temperatures, has an A- hexagonal crystal structure. The metal atoms are surrounded by a 7 coordinate group of atoms, the oxygen ions are in an octahedral shape around the metal atom and there is one oxygen ion above ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
World Scientific
World Scientific Publishing is an academic publisher of scientific, technical, and medical books and journals headquartered in Singapore. The company was founded in 1981. It publishes about 600 books annually, along with 135 journals in various fields. In 1995, World Scientific co-founded the London-based Imperial College Press together with the Imperial College of Science, Technology and Medicine. Company structure The company head office is in Singapore. The Chairman and Editor-in-Chief is Dr Phua Kok Khoo, while the Managing Director is Doreen Liu. The company was co-founded by them in 1981. Imperial College Press In 1995 the company co-founded Imperial College Press, specializing in engineering, medicine and information technology, with Imperial College London. In 2006, World Scientific assumed full ownership of Imperial College Press, under a license granted by the university. Finally, in August 2016, ICP was fully incorporated into World Scientific under the new imprint ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |