|
Superheated Steam
Superheated steam is steam at a temperature higher than its vaporization point at the absolute pressure where the temperature is measured. Superheated steam can therefore cool (lose internal energy) by some amount, resulting in a lowering of its temperature without changing state (i.e., condensing) from a gas to a mixture of saturated vapor and liquid. If unsaturated steam (a mixture which contains both water vapor and liquid water droplets) is heated at constant pressure, its temperature will also remain constant as the vapor quality (think dryness, or percent saturated vapor) increases towards 100%, and becomes dry (i.e., no saturated liquid) saturated steam. Continued heat input will then "super" heat the dry saturated steam. This will occur if saturated steam contacts a surface with a higher temperature. Superheated steam and liquid water cannot coexist under thermodynamic equilibrium, as any additional heat simply evaporates more water and the steam will become saturate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
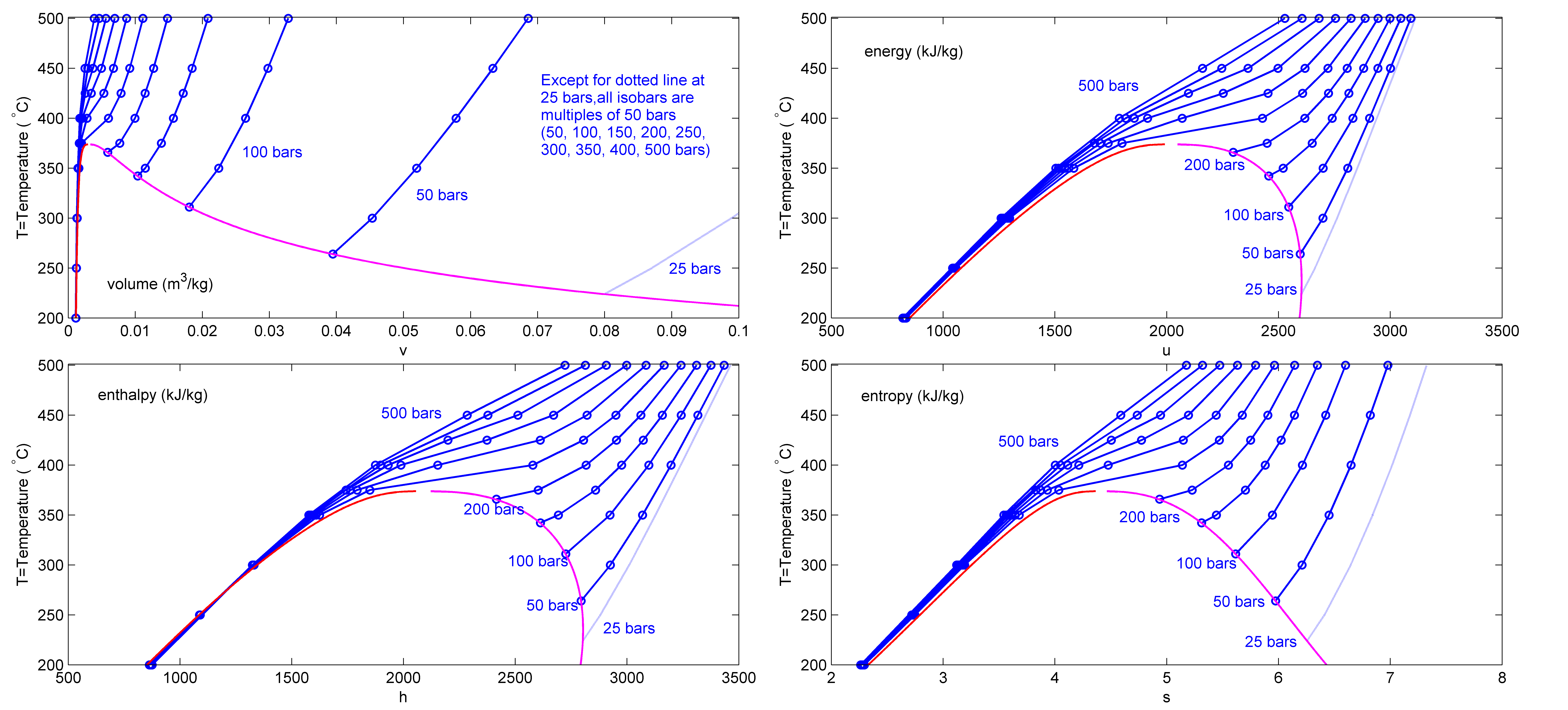
Superheated Steam Table Fits On 2 Pages
A superheater is a device used to convert saturated steam or wet steam into superheated steam or dry steam. Superheated steam is used in steam turbines for electricity generation, in some steam engines, and in processes such as steam reforming. There are three types of superheaters: radiant, convection, and separately fired. A superheater can vary in size from a few tens of feet to several hundred feet (a few metres to some hundred metres). Types * A radiant superheater is placed directly in the radiant zone of the combustion chamber near the water wall so as to absorb heat by radiation. * A convection superheater is located in the convective zone of the furnace, in the path of the hot flue gases, usually ahead of an economizer. A convection superheater is also called a primary superheater. * A separately fired superheater is a superheater that is placed outside the main boiler and has its own separate combustion system. This superheater design incorporates additional burners i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radiation
In physics, radiation is the emission or transmission of energy in the form of waves or particles through space or a material medium. This includes: * ''electromagnetic radiation'' consisting of photons, such as radio waves, microwaves, infrared, visible light, ultraviolet, x-rays, and Gamma ray, gamma radiation (γ) * ''particle radiation'' consisting of particles of non-zero rest energy, such as alpha radiation (α), beta radiation (β), proton radiation and neutron radiation * ''acoustics, acoustic radiation'', such as ultrasound, sound, and seismic waves, all dependent on a physical transmission medium * ''gravitational radiation'', in the form of gravitational waves, ripples in spacetime Radiation is often categorized as either ''ionizing radiation, ionizing'' or ''non-ionizing radiation, non-ionizing'' depending on the energy of the radiated particles. Ionizing radiation carries more than 10 electron volt, electron volts (eV), which is enough to ionize atoms and molecul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vapor–liquid Equilibrium
In thermodynamics and chemical engineering, the vapor–liquid equilibrium (VLE) describes the distribution of a chemical species between the vapor phase and a liquid phase. The Vapor quality, concentration of a vapor in contact with its liquid, especially at Thermodynamic equilibrium, equilibrium, is often expressed in terms of vapor pressure, which will be a partial pressure (a part of the total gas pressure) if any other gas(es) are present with the vapor. The equilibrium vapor pressure of a liquid is in general strongly dependent on temperature. At vapor–liquid equilibrium, a liquid with individual components in certain concentrations will have an equilibrium vapor in which the concentrations or partial pressures of the vapor components have certain values depending on all of the liquid component concentrations and the temperature. The converse is also true: if a vapor with components at certain concentrations or partial pressures is in vapor–liquid equilibrium with its ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steam Turbine
A steam turbine or steam turbine engine is a machine or heat engine that extracts thermal energy from pressurized steam and uses it to do mechanical work utilising a rotating output shaft. Its modern manifestation was invented by Sir Charles Parsons in 1884. It revolutionized marine propulsion and navigation to a significant extent. Fabrication of a modern steam turbine involves advanced metalwork to form high-grade steel alloys into precision parts using technologies that first became available in the 20th century; continued advances in durability and efficiency of steam turbines remains central to the energy economics of the 21st century. The largest steam turbine ever built is the 1,770 MW Arabelle steam turbine built by Arabelle Solutions (previously GE Steam Power), two units of which will be installed at Hinkley Point C Nuclear Power Station, England. The steam turbine is a form of heat engine that derives much of its improvement in thermodynamic efficiency from the u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boiling Point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. The boiling point of a liquid varies depending upon the surrounding environmental pressure. A liquid in a partial vacuum, i.e., under a lower pressure, has a lower boiling point than when that liquid is at atmospheric pressure. Because of this, water boils at 100°C (or with scientific precision: ) under standard pressure at sea level, but at at altitude. For a given pressure, different liquids will boiling, boil at different temperatures. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals the defined atmospheric pressure at sea level, one Atmosphere (unit), atmosphere. At that temperature, the vapor pressure of the liquid becomes sufficient to overcome atmospheric pre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Incompressible Flow
In fluid mechanics, or more generally continuum mechanics, incompressible flow is a flow in which the material density does not vary over time. Equivalently, the divergence of an incompressible flow velocity is zero. Under certain conditions, the flow of compressible fluids can be modelled as incompressible flow to a good approximation. Derivation The fundamental requirement for incompressible flow is that the density, \rho , is constant within a small element volume, ''dV'', which moves at the flow velocity u. Mathematically, this constraint implies that the material derivative (discussed below) of the density must vanish to ensure incompressible flow. Before introducing this constraint, we must apply the conservation of mass to generate the necessary relations. The mass is calculated by a volume integral of the density, \rho : : = . The conservation of mass requires that the time derivative of the mass inside a control volume be equal to the mass flux, J, acro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reciprocating Engine
A reciprocating engine, more often known as a piston engine, is a heat engine that uses one or more reciprocating pistons to convert high temperature and high pressure into a rotating motion. This article describes the common features of all types. The main types are: the internal combustion engine, used extensively in motor vehicles; the steam engine, the mainstay of the Industrial Revolution; and the Stirling engine for niche applications. Internal combustion engines are further classified in two ways: either a spark-ignition (SI) engine, where the spark plug initiates the combustion; or a compression-ignition (CI) engine, where the air within the cylinder is compressed, thus heating it, so that the heated air ignites fuel that is injected then or earlier.''Thermodynamics: An Engineering Approach'' by Yunus A. Cengal and Michael A. Boles Common features in all types There may be one or more pistons. Each piston is inside a cylinder, into which a gas is introduced, e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steam Turbine
A steam turbine or steam turbine engine is a machine or heat engine that extracts thermal energy from pressurized steam and uses it to do mechanical work utilising a rotating output shaft. Its modern manifestation was invented by Sir Charles Parsons in 1884. It revolutionized marine propulsion and navigation to a significant extent. Fabrication of a modern steam turbine involves advanced metalwork to form high-grade steel alloys into precision parts using technologies that first became available in the 20th century; continued advances in durability and efficiency of steam turbines remains central to the energy economics of the 21st century. The largest steam turbine ever built is the 1,770 MW Arabelle steam turbine built by Arabelle Solutions (previously GE Steam Power), two units of which will be installed at Hinkley Point C Nuclear Power Station, England. The steam turbine is a form of heat engine that derives much of its improvement in thermodynamic efficiency from the u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
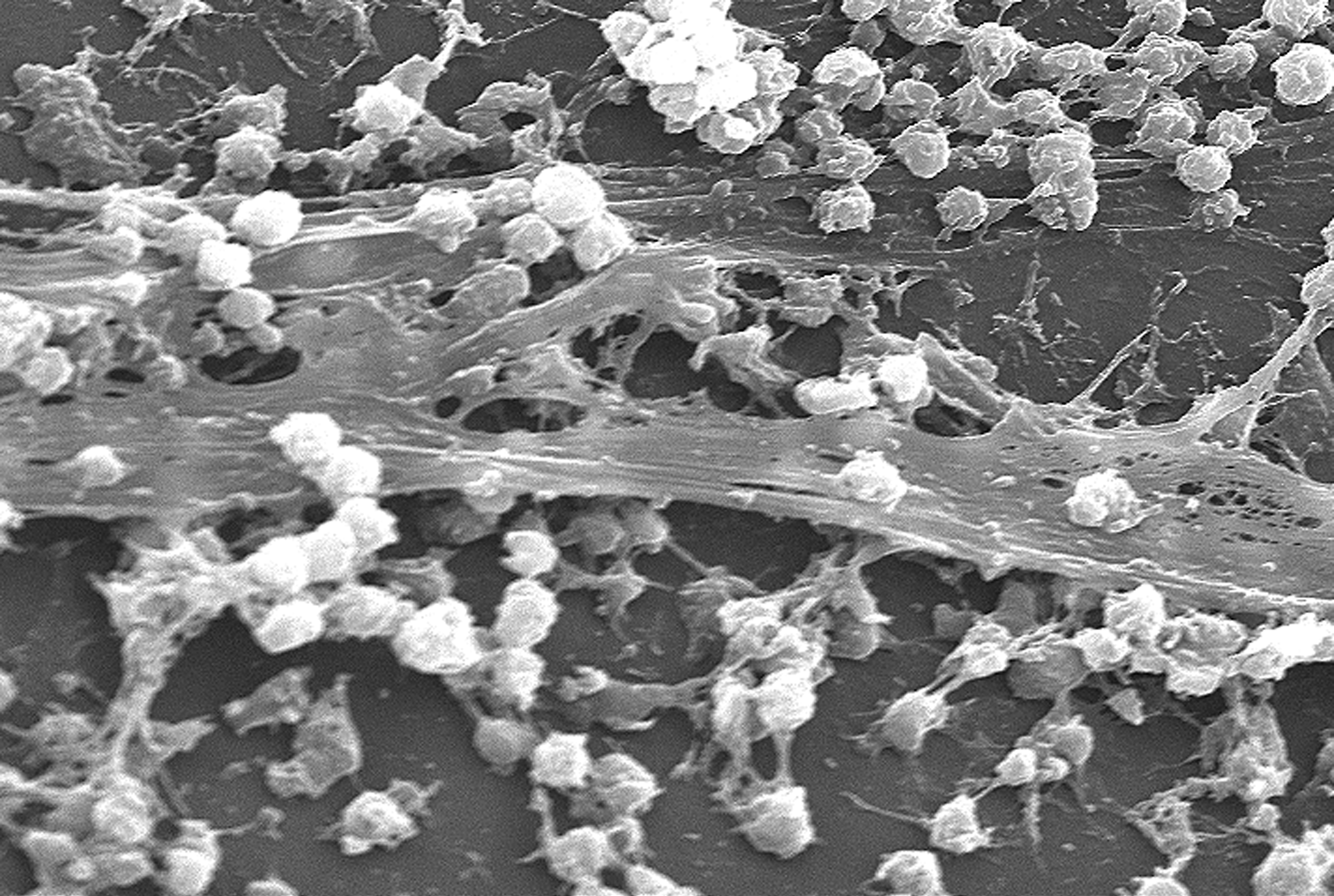
Biofilm
A biofilm is a Syntrophy, syntrophic Microbial consortium, community of microorganisms in which cell (biology), cells cell adhesion, stick to each other and often also to a surface. These adherent cells become embedded within a slimy extracellular matrix that is composed of extracellular polymeric substances (EPSs). The cells within the biofilm produce the EPS components, which are typically a polymeric combination of extracellular polysaccharides, proteins, lipids and DNA. Because they have a three-dimensional structure and represent a community lifestyle for microorganisms, they have been metaphorically described as "cities for microbes". Biofilms may form on living (biotic) or non-living (abiotic) surfaces and can be common in natural, industrial, and hospital settings. They may constitute a microbiome or be a portion of it. The microbial cells growing in a biofilm are physiology, physiologically distinct from planktonic cells of the same organism, which, by contrast, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saturated Steam
Steam is water vapor, often mixed with air or an aerosol of liquid water droplets. This may occur due to evaporation or due to boiling, where heat is applied until water reaches the enthalpy of vaporization. Saturated or superheated steam is invisible; however, wet steam, a visible mist or aerosol of water droplets, is often referred to as "steam". When liquid water becomes steam, it increases in volume by 1,700 times at standard temperature and pressure; this change in volume can be converted into mechanical work by steam engines such as reciprocating piston type engines and steam turbines, which are a sub-group of steam engines. Piston type steam engines played a central role in the Industrial Revolution and modern steam turbines are used to generate more than 80% of the world's electricity. If liquid water comes in contact with a very hot surface or depressurizes quickly below its vapour pressure, it can create a steam explosion. Types of steam and conversions Steam i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Insulation
Thermal insulation is the reduction of heat transfer (i.e., the transfer of thermal energy between objects of differing temperature) between objects in thermal contact or in range of radiative influence. Thermal insulation can be achieved with specially engineered methods or processes, as well as with suitable object shapes and materials. Heat flow is an inevitable consequence of contact between objects of different temperature. Thermal insulation provides a region of insulation in which thermal conduction is reduced, creating a thermal break or thermal barrier, or thermal radiation is reflected rather than absorbed by the lower-temperature body. The insulating capability of a material is measured as the inverse of thermal conductivity, thermal conductivity (k). Low thermal conductivity is equivalent to high insulating capability (R-value (insulation), resistance value). In thermal engineering, other important properties of insulating materials are product density, density (ρ) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Transfer Coefficient
In thermodynamics, the heat transfer coefficient or film coefficient, or film effectiveness, is the Proportional (mathematics), proportionality constant between the heat flux and the thermodynamic driving force for the Heat transfer, flow of heat (i.e., the Temperature gradient, temperature difference, ). It is used to calculate heat transfer between components of a system; such as by convection between a fluid and a solid. The heat transfer coefficient has SI units in Watt, watts per square meter per kelvin (W/(m2K)). The overall heat transfer rate for combined modes is usually expressed in terms of an overall Thermal conduction, conductance or heat transfer coefficient, . Upon reaching a steady state of flow, the heat transfer rate is: :\dot=hA(T_2-T_1) where (in SI units): : \dot: Heat transfer rate (W) : h: Heat transfer coefficient (W/m2K) : A: surface area where the heat transfer takes place (m2) : T_2: temperature of the surrounding fluid (K) : T_1: temperature of the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |