|
Kelch Repeat
The Kelch motif is a region of protein sequence found widely in proteins from bacteria and eukaryotes. This sequence motif is composed of about 50 amino acid residues which form a structure of a four stranded beta-sheet "blade". This sequence motif is found in between five and eight tandem copies per protein which fold together to form a larger circular solenoid structure called a beta-propeller domain. Proteins containing Kelch motifs The Kelch motif is widely found in eukaryotic and bacterial species. Notably the human genome contains around 100 proteins containing the Kelch motif. Within individual proteins the motif occurs multiple times. For example, the motif appears 6 times in ''Drosophila'' egg-chamber regulatory protein. The motif is also found in mouse protein MIPP and in a number of poxviruses. In addition, kelch repeats have been recognised in alpha- and beta-scruin, in galactose oxidase from the fungus '' Dactylium dendroides'' and in the ''Escherichia coli'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Sequence
Protein primary structure is the linear sequence of amino acids in a peptide or protein. By convention, the primary structure of a protein is reported starting from the amino-terminal (N) end to the carboxyl-terminal (C) end. Protein biosynthesis is most commonly performed by ribosomes in cells. Peptides can also be synthesized in the laboratory. Protein primary structures can be directly sequenced, or inferred from DNA sequences. Formation Biological Amino acids are polymerised via peptide bonds to form a long backbone, with the different amino acid side chains protruding along it. In biological systems, proteins are produced during translation by a cell's ribosomes. Some organisms can also make short peptides by non-ribosomal peptide synthesis, which often use amino acids other than the standard 20, and may be cyclised, modified and cross-linked. Chemical Peptides can be synthesised chemically via a range of laboratory methods. Chemical methods typically synthesise ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Poxviruses
''Poxviridae'' is a family of double-stranded DNA viruses. Vertebrates and arthropods serve as natural hosts. There are currently 83 species in this family, divided among 22 genera, which are divided into two subfamilies. Diseases associated with this family include smallpox. Four genera of poxviruses may infect humans: ''Orthopoxvirus'', ''Parapoxvirus'', ''Yatapoxvirus'', ''Molluscipoxvirus''. ''Orthopoxvirus'': smallpox virus (variola), vaccinia virus, cowpox virus, monkeypox virus; ''Parapoxvirus'': orf virus, pseudocowpox, bovine papular stomatitis virus; ''Yatapoxvirus'': tanapox virus, yaba monkey tumor virus; ''Molluscipoxvirus'': molluscum contagiosum virus (MCV). The most common are vaccinia (seen on the Indian subcontinent) and molluscum contagiosum, but monkeypox infections are rising (seen in west and central African rainforest countries). The similarly named disease chickenpox is not a true poxvirus and is caused by the herpesvirus varicella zoster. Etymology ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
WD40 Repeat
The WD40 repeat (also known as the WD or beta-transducin repeat) is a short structural motif of approximately 40 amino acids, often terminating in a tryptophan-aspartic acid (W-D) dipeptide. Tandem copies of these repeats typically fold together to form a type of circular solenoid protein domain called the WD40 domain. Structure WD40 domain-containing proteins have 4 to 16 repeating units, all of which are thought to form a circularised beta-propeller structure (see figure to the right). The WD40 domain is composed of several repeats, a variable region of around 20 residues at the beginning followed by a more common repeated set of residues. These repeats typically form a four stranded anti-parallel beta sheet or blade. These blades come together to form a propeller with the most common being a 7 bladed beta propeller. The blades interlock so that the last beta strand of one repeat forms with the first three of the next repeat to form the 3D blade structure. Function WD40-repe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
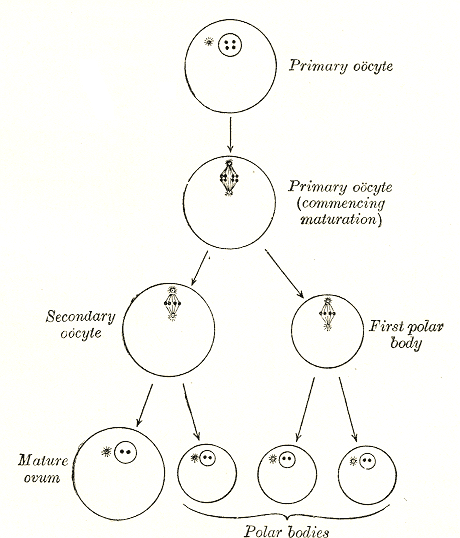
Oocyte
An oocyte (, ), oöcyte, or ovocyte is a female gametocyte or germ cell involved in reproduction. In other words, it is an immature ovum, or egg cell. An oocyte is produced in a female fetus in the ovary during female gametogenesis. The female germ cells produce a primordial germ cell (PGC), which then undergoes mitosis, forming oogonia. During oogenesis, the oogonia become primary oocytes. An oocyte is a form of genetic material that can be collected for cryoconservation. Formation The formation of an oocyte is called oocytogenesis, which is a part of oogenesis. Oogenesis results in the formation of both primary oocytes during fetal period, and of secondary oocytes after it as part of ovulation. Characteristics Cytoplasm Oocytes are rich in cytoplasm, which contains yolk granules to nourish the cell early in development. Nucleus During the primary oocyte stage of oogenesis, the nucleus is called a germinal vesicle. The only normal human type of secondary oocyte has t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neuraminidase
Exo-α-sialidase (EC 3.2.1.18, sialidase, neuraminidase; systematic name acetylneuraminyl hydrolase) is a glycoside hydrolase that cleaves the glycosidic linkages of neuraminic acids: : Hydrolysis of α-(2→3)-, α-(2→6)-, α-(2→8)- glycosidic linkages of terminal sialic acid residues in oligosaccharides, glycoproteins, glycolipids, colominic acid and synthetic substrates Neuraminidase enzymes are a large family, found in a range of organisms. The best-known neuraminidase is the viral neuraminidase, a drug target for the prevention of the spread of influenza infection. The viral neuraminidases are frequently used as antigenic determinants found on the surface of the influenza virus. Some variants of the influenza neuraminidase confer more virulence to the virus than others. Other homologues are found in mammalian cells, which have a range of functions. At least four mammalian sialidase homologues have been described in the human genome (see NEU1, NEU2, NEU3, NEU4). Sialidas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Galactose Oxidase
Galactose oxidase (D-galactose:oxygen 6-oxidoreductase, D-galactose oxidase, beta-galactose oxidase; abbreviated GAO, GAOX, GOase; ) is an enzyme that catalyzes the oxidation of D-galactose in some species of fungi. Galactose oxidase belongs to the family of oxidoreductases. Copper ion is required as a cofactor for galactose oxidase. A remarkable feature of galactose oxidase is that it is a free radical enzyme. Its catalytic site contains a free radical ligand coordinating to the copper center. This free radical ligand is a covalently cross-linked cysteine and tyrosine side chains that is formed during post-translational modification. Background Found in several fungal species such as ''Fusarium graminearum'' NRRL 2903 (formerly misidentified as ''Dactylium dendroides''), and other species of Fusarium and Aspergillus genera, galactose oxidase was first isolated in 1959. This enzyme is secreted by fungi to function in extracellular space. Although the oxidation reaction of D-gala ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sialic Acid Mutarotase
N-acetylneuraminate epimerase (, ''sialic acid epimerase'', ''N-acetylneuraminate mutarotase'', sialic acid mutarotase, ''YjhT'', NanM) is an enzyme with systematic name ''N-acetyl-alpha-neuraminate 2-epimerase''. This enzyme catalyses the following chemical reaction : ''N''-acetyl-alpha-neuraminate \rightleftharpoons ''N''-acetyl-beta-neuraminate Sialoglycoconjugates present in vertebrates are linked exclusively by alpha-linkages. See also * sialic acid ** ''N''-acetylneuraminate * Mutarotation Mutarotation is the change in the ''optical rotation'' because of the change in the equilibrium between two anomers, when the corresponding stereocenters interconvert. Cyclic sugars show mutarotation as α and β anomeric forms interconvert. The op ... References External links * {{Portal bar, Biology, border=no EC 5.1.3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Escherichia Coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escherichia'' that is commonly found in the lower intestine of warm-blooded organisms. Most ''E. coli'' strains are harmless, but some serotypes ( EPEC, ETEC etc.) can cause serious food poisoning in their hosts, and are occasionally responsible for food contamination incidents that prompt product recalls. Most strains do not cause disease in humans and are part of the normal microbiota of the gut; such strains are harmless or even beneficial to humans (although these strains tend to be less studied than the pathogenic ones). For example, some strains of ''E. coli'' benefit their hosts by producing vitamin K2 or by preventing the colonization of the intestine by pathogenic bacteria. These mutually beneficial relationships between ''E. col ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MIPP
The enzyme multiple inositol-polyphosphate phosphatase (EC 3.1.3.62) catalyzes the reaction :''myo''-inositol hexakisphosphate + H2O \rightleftharpoons ''myo''-inositol pentakisphosphate (mixed isomers) + phosphate This enzyme belongs to the family of hydrolases, specifically those acting on phosphoric monoester bonds. The systematic name is 1D-''myo''-inositol-hexakisphosphate 5-phosphohydrolase. Other names in common use include inositol (1,3,4,5)-tetrakisphosphate 3-phosphatase, inositol 1,3,4,5-tetrakisphosphate 3-phosphomonoesterase, inositol 1,3,4,5-tetrakisphosphate-5-phosphomonoesterase, inositol tetrakisphosphate phosphomonoesterase, inositol-1,3,4,5-tetrakisphosphate 3-phosphatase, and MIPP. This enzyme participates in inositol phosphate metabolism Inositol phosphates are a group of mono- to hexaphosphorylated inositols. They play crucial roles in diverse cellular functions, such as cell growth, apoptosis, cell migration, endocytosis, and cell differentiation. The g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among the first life forms to appear on Earth, and are present in most of its habitats. Bacteria inhabit soil, water, acidic hot springs, radioactive waste, and the deep biosphere of Earth's crust. Bacteria are vital in many stages of the nutrient cycle by recycling nutrients such as the fixation of nitrogen from the atmosphere. The nutrient cycle includes the decomposition of dead bodies; bacteria are responsible for the putrefaction stage in this process. In the biological communities surrounding hydrothermal vents and cold seeps, extremophile bacteria provide the nutrients needed to sustain life by converting dissolved compounds, such as hydrogen sulphide and methane, to energy. Bacteria also live in symbiotic and parasitic relationsh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |