Galactose Oxidase on:
[Wikipedia]
[Google]
[Amazon]
Galactose oxidase (D-galactose:oxygen 6-oxidoreductase, D-galactose oxidase, beta-galactose oxidase; abbreviated GAO, GAOX, GOase; ) is an
 Galactose oxidase is a type II copper protein. It contains a single copper center that adopts square planar or square-based pyramidal
Galactose oxidase is a type II copper protein. It contains a single copper center that adopts square planar or square-based pyramidal

enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
that catalyzes the oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
of D-galactose in some species of fungi
A fungus ( : fungi or funguses) is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and molds, as well as the more familiar mushrooms. These organisms are classified as a kingdom, separately from ...
.
Galactose oxidase belongs to the family of oxidoreductases
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule, the reductant, also called the electron donor, to another, the oxidant, also called the electron acceptor. This group of enzymes usually uti ...
. Copper ion is required as a cofactor for galactose oxidase. A remarkable feature of galactose oxidase is that it is a free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Ailments of unknown cause
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabo ...
enzyme. Its catalytic site
In biology and biochemistry, the active site is the region of an enzyme where Enzyme substrate, substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid, amino acid residues that form temporary bonds with t ...
contains a free radical ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
coordinating to the copper center. This free radical ligand is a covalently cross-linked cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile.
When present as a deprotonated catalytic residue, sometime ...
and tyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Gr ...
side chains that is formed during post-translational modification
Post-translational modification (PTM) is the covalent and generally enzymatic modification of proteins following protein biosynthesis. This process occurs in the endoplasmic reticulum and the golgi apparatus. Proteins are synthesized by ribosome ...
.
Background
Found in several fungal species such as ''Fusarium graminearum
''Gibberella zeae'', also known by the name of its anamorph ''Fusarium graminearum'', is a fungal plant pathogen which causes fusarium head blight (FHB), a devastating disease on wheat and barley. The pathogen is responsible for billions of dolla ...
'' NRRL 2903 (formerly misidentified as ''Dactylium dendroides''), and other species of Fusarium
''Fusarium'' is a large genus of filamentous fungi, part of a group often referred to as hyphomycetes, widely distributed in soil and associated with plants. Most species are harmless saprobes, and are relatively abundant members of the soil mi ...
and Aspergillus
' () is a genus consisting of several hundred mold species found in various climates worldwide.
''Aspergillus'' was first catalogued in 1729 by the Italian priest and biologist Pier Antonio Micheli. Viewing the fungi under a microscope, Miche ...
genera
Genus ( plural genera ) is a taxonomic rank used in the biological classification of living and fossil organisms as well as viruses. In the hierarchy of biological classification, genus comes above species and below family. In binomial nomenclat ...
, galactose oxidase was first isolated in 1959. This enzyme is secreted by fungi to function in extracellular space. Although the oxidation reaction of D-galactose gives galactose oxidase its name, the coupled reduction of dioxygen
There are several known allotropes of oxygen. The most familiar is molecular oxygen (O2), present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone (O3). Others are:
*A ...
to hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%� ...
is believed to have greater physiological significance in yeasts. Hydrogen peroxide which can be produced by yeasts in this way is possibly a bacteriostatic agent
A bacteriostatic agent or bacteriostat, abbreviated Bstatic, is a biological or chemical agent that stops bacteria from reproducing, while not necessarily killing them otherwise. Depending on their application, bacteriostatic antibiotics, disinfect ...
.
Protein structure
Galactose oxidase contains 639amino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
. It is a single peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides.
A ...
monomer
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
Mo ...
that has three β-structural domains. Domain 1 (residues 1-155) is a β-sandwich
Beta-sandwich, β-sandwich domains consisting of 80 to 350 amino acids occur commonly in proteins. They are characterized by two opposing antiparallel beta sheets (β-sheets). The number of strands found in such domains may differ from one protein ...
consisting of eight antiparallel β-strands. It contains a possible binding site for Na+ or Ca2+, which may serve structural roles in the protein. Another feature of Domain 1 is the presence of a carbohydrate binding site that direct the enzyme to bind to extracellular carbohydrates. Domain 2 (residues 156-552) contains the copper binding site. The β-strands in Domain 2 are organized as a seven-fold propeller, and each of the seven structural units is a subdomain consisting of four antiparallel β-strands. Domain 3 (residues 553-639) consists of seven anti-parallel β-strands and forms a “cap” over Domain 2. One histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the de ...
(His581) of Domain 3 serves as the ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
for copper, contributing to the metal-containing active site of the enzyme.
Active site
 Galactose oxidase is a type II copper protein. It contains a single copper center that adopts square planar or square-based pyramidal
Galactose oxidase is a type II copper protein. It contains a single copper center that adopts square planar or square-based pyramidal coordination geometry
The term coordination geometry is used in a number of related fields of chemistry and solid state chemistry/physics.
Molecules
The coordination geometry of an atom is the geometrical pattern formed by atoms around the central atom.
Inorganic coo ...
. The copper center has five coordinating ligands: two tyrosines
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Gr ...
(Tyr272 and Tyr495), two histidines (His496 and His581), and a solvent molecule that is usually water. The copper in the active site of galactose oxidase is described as having a "distorted square pyramidal" coordination geometry. Tyr495 is the axial ligand, the other four ligands lie roughly in a plane. Both histidines coordinate with copper through 3-nitrogen. Copper-H2O bond is the longest coordinate bond
In coordination chemistry, a coordinate covalent bond, also known as a dative bond, dipolar bond, or coordinate bond is a kind of two-center, two-electron covalent bond in which the two electrons derive from the same atom. The bonding of metal io ...
; it is labile
Lability refers to something that is constantly undergoing change or is likely to undergo change.
Biochemistry
In reference to biochemistry, this is an important concept as far as kinetics is concerned in metalloproteins. This can allow for th ...
and can be replaced by a substrate molecule. Tyr272 forms a dimer
Dimer may refer to:
* Dimer (chemistry), a chemical structure formed from two similar sub-units
** Protein dimer, a protein quaternary structure
** d-dimer
* Dimer model, an item in statistical mechanics, based on ''domino tiling''
* Julius Dimer ( ...
with a cysteine (Cys228) through an ortho carbon of tyrosine and the sulfur atom of cysteine, which is supported by X-ray crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles ...
studies. The Tyr-Cys cross-link decreases the structural flexibility of Tyr272. This cross-linked tyrosinate is also a free radical. In the fully oxidized form of galactose oxidase, the free radical couples to the copper(II) center antiferromagnetically, supported by EPR spectroscopic studies. Moreover, the formation of cross-linking thioether bond is believed to lower the oxidation potential
Redox potential (also known as oxidation / reduction potential, ''ORP'', ''pe'', ''E_'', or E_) is a measure of the tendency of a chemical species to acquire electrons from or lose electrons to an electrode and thereby be reduced or oxidised respe ...
of Tyr272 phenoxide
Phenolates (also called phenoxides) are anions, salts, and esters of phenols. They may be formed by reaction of phenols with strong base.
Properties
Alkali metal phenolates, such as sodium phenolate hydrolyze in aqueous solution to form basic s ...
, making this phenoxyl more easily oxidized to form the radical in post-translational modification.
The free radical in galactose oxidase is unusually stable compared to many other protein free radicals. The free radical ligand is stabilized mainly in two ways. Firstly, as revealed by computational chemistry
Computational chemistry is a branch of chemistry that uses computer simulation to assist in solving chemical problems. It uses methods of theoretical chemistry, incorporated into computer programs, to calculate the structures and properties of m ...
studies, the unpaired electron is stabilized through delocalization
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref>
The term delocalization is general and can have slightly diff ...
by the aromatic ring
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to saturat ...
of tyrosine and the cross-linked cysteine sulfur, with the oxygen atom of Tyr272 possessing high unpaired electron density
In quantum chemistry, electron density or electronic density is the measure of the probability of an electron being present at an infinitesimal element of space surrounding any given point. It is a scalar quantity depending upon three spatial va ...
. Some experimental evidence also suggests that axial Tyr495 is also involved in unpaired electron delocalization. Secondly, the indole
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environmen ...
ring of a tryptophan
Tryptophan (symbol Trp or W)
is an α-amino acid that is used in the biosynthesis of proteins. Tryptophan contains an α-amino group, an α- carboxylic acid group, and a side chain indole, making it a polar molecule with a non-polar aromatic ...
(Trp290) lies above and parallel to Tyrosine-Cysteine, behaving like a shield protecting the radical from the external solvent environment. Supporting evidence comes from that mutation
In biology, a mutation is an alteration in the nucleic acid sequence of the genome of an organism, virus, or extrachromosomal DNA. Viral genomes contain either DNA or RNA. Mutations result from errors during DNA or viral replication, mi ...
of this tryptophan residue leads to a lower stability of the active form of galactose oxidase. Additionally, the outer sphere of the active site consists of many aromatic residues that give the active site a hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, th ...
character. There are also extensive hydrogen bonding
In chemistry, a hydrogen bond (or H-bond) is a primarily electrostatic force of attraction between a hydrogen (H) atom which is covalently bound to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a l ...
networks surround the active site.
Reaction
In yeasts, galactose oxidase catalyzes the following reaction: :D-galactose + O2 D-galacto-hexodialdose + H2O2 This reaction is essentially the oxidation of primaryalcohol
Alcohol most commonly refers to:
* Alcohol (chemistry), an organic compound in which a hydroxyl group is bound to a carbon atom
* Alcohol (drug), an intoxicant found in alcoholic drinks
Alcohol may also refer to:
Chemicals
* Ethanol, one of sev ...
using dioxygen
There are several known allotropes of oxygen. The most familiar is molecular oxygen (O2), present at significant levels in Earth's atmosphere and also known as dioxygen or triplet oxygen. Another is the highly reactive ozone (O3). Others are:
*A ...
to form the corresponding aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
and hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%� ...
. It has been shown that galactose oxidase is also able to catalyze various primary alcohols other than galactose. In fact, galactose oxidase catalyzes dihydroxyacetone
Dihydroxyacetone (; DHA), also known as glycerone, is a simple saccharide (a triose) with formula .
DHA is primarily used as an ingredient in sunless tanning products. It is often derived from plant sources such as sugar beets and sugar cane, an ...
three times faster than it does to galactose. The reaction is regioselective
In chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base ...
, in that it cannot oxidize secondary alcohol.
This two-electron oxidation is achieved by the double-redox site: the copper(II) metal center and the free radical, each capable of accepting one electron from the substrate. This double-redox center has three accessible oxidation levels. In the catalytic cycle of galactose oxidase, the enzyme shuttles between the fully oxidized form and the fully reduced form. The semi-oxidized form is the inactive form.

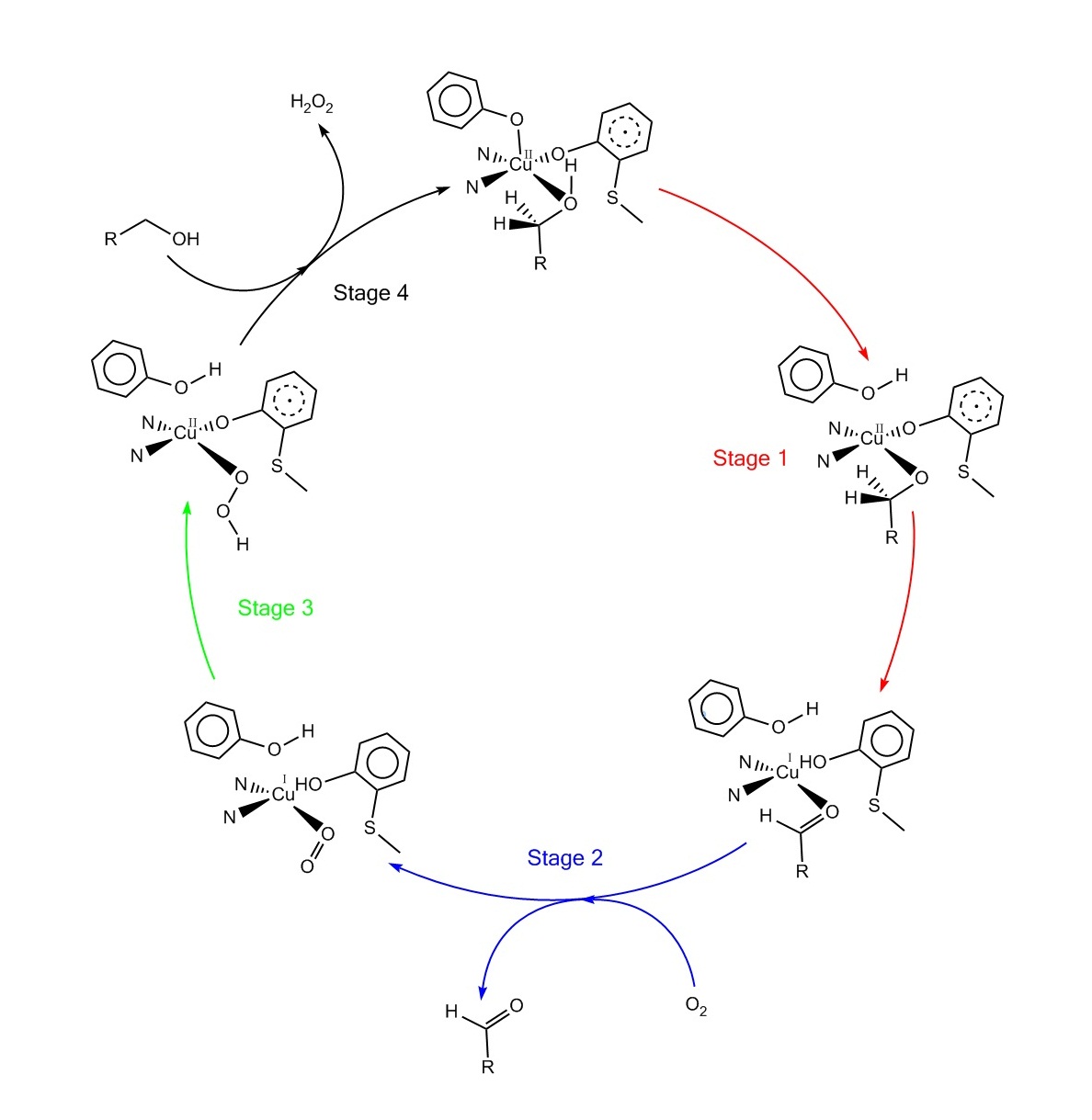
Catalytic mechanism
The accepted catalytic mechanism, called the “ping-pong mechanism,” consists of four major stages. The first stage is the oxidation of the substrate by the double-redox center. After thehydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
group of substrate alcohol occupies the solvent coordination site, the hydroxyl group is deprotonated by Tyr495, followed by the release of Tyr495. This step makes the alcohol more prone to oxidation. The proton on the carbon to which the hydroxyl group used to be attached is then transferred to Tyr272 (serving as the hydrogen acceptor), coupled with the oxidation of the substrate. One electron goes to the radical ligand, the other electron goes to the copper(II) center, which is then reduced to copper(I) as a result. Meanwhile, Tyr272 radical is also reduced. The proton subtraction step is rate determining and stereospecific
In chemistry, stereospecificity is the property of a reaction mechanism that leads to different stereoisomeric reaction products from different stereoisomeric reactants, or which operates on only one (or a subset) of the stereoisomers."Overlap Con ...
since only the pro-S hydrogen on the alcohol carbon is removed (supported by studies of its kinetic isotope effect). The overall result of stage 1 is the removal of two hydrogen atoms and the removal two electrons from the substrate, of which the order is unclear, however. The second stage is the release of oxidized substrate (aldehyde in this case) and the coordination of dioxygen at the substrate coordination site. In the third stage, dioxygen is rapidly reduced by copper(I) to form superoxide
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula . The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of the ...
. The superoxide is a reactive species that subtracts the proton and an electron from the Tyr272 and re-forms the tyrosine radical. In the fourth stage, the hydroperoxide deprotonates Tyr496 and is released as H2O2. Subsequent axial coordination of Tyr496 and equatorial coordination of new substrate molecule to the copper center completes the turnover of the enzyme.

Post-translational modification
Prepro-GAOX (galactose oxidase with signal sequence) is processed twice byproteolytic cleavage
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, ...
in the leader sequence to form the mature GAOX peptide (pro-GAOX). The first cleavage removes a sequence of 24 amino acids by signal peptidase
Signal peptidases are enzymes that convert secretory and some membrane proteins to their mature or pro forms by cleaving their signal peptides from their N-termini.
Signal peptidases were initially observed in endoplasmic reticulum (ER)-der ...
. The second cleavage removes another sequence of 17 amino acids.
The covalent linkage between Tyr272 and Cys228 forms after pro
Pro is an abbreviation meaning "professional".
Pro, PRO or variants thereof may also refer to:
People
* Miguel Pro (1891–1927), Mexican priest
* Pro Hart (1928–2006), Australian painter
* Mlungisi Mdluli (born 1980), South African retired f ...
-GAOX has been made. The occurrence of this modification does not seem to require any other “helper” proteins. The current mechanism for the formation of this covalent linkage suggests the requirement of copper(I) and dioxygen. The mechanism for this tyrosine-cysteine linkage is not thoroughly understood, but a few key events have been predicted: copper(I) coordinates with Tyr272 and histidines at the (future) active site. Reaction of dioxygen with the active site complex generates a free radical intermediate. Two possible forms of the free radical, thiyl and phenoxyl
In chemistry, the alkoxy group is an alkyl group which is singularly bonded to oxygen; thus . The range of alkoxy groups is vast, the simplest being methoxy (). An ethoxy group () is found in the organic compound ethyl phenyl ether (, also kn ...
, are possible; addition of thiyl radical to phenol, or addition of phenoxyl radical to thiol, generates the covalent linkage between the sulfur atom of cysteine and the aromatic ring of tyrosine; A second dioxygen molecule reacts with the copper center coordinated with cross-linked tyrosine-cysteine to generate radical-copper complex.
Applications
Bioanalysis
Biomolecules in samples such as galactose can be quantified using oxygen detection method, since one equivalent consumption of oxygen corresponds to one equivalent primary hydroxyl group oxidized. The formation of hydrogen peroxide during substrate oxidation can also be used forcolorimetric
Colorimetry is "the science and technology used to quantify and describe physically the human color perception".
It is similar to spectrophotometry, but is distinguished by its interest in reducing spectra to the physical correlates of color ...
detection of galactose using dyes that are oxidized by hydrogen peroxide. Because carbohydrates can normally have primary hydroxyl groups, galactose oxidase can be used to modify cell surface glycoproteins
Glycoproteins are proteins which contain oligosaccharide chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycos ...
to achieve cell labelling.
Organic synthesis
Galactose oxidase has been utilized as abiocatalyst
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
in the synthesis of aldehydes
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group ...
and carboxylic acids
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
from primary alcohols.
Biomimetic compounds
Our understanding of the mechanism of galactose oxidase inspires researchers to develop model compounds that mimics the structure and function of galactose oxidase. It appears that electron-sharing between the copper and the free radical is the crucial element in the success of synthesizing these compounds. The first model compound of GAOX made is u(II)(dnc) which utilizes duncamine (dnc) as thechelating ligand
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are ...
. Other model compounds have been studied and reported in literature.
References
{{Portal bar, Biology, border=no EC 1.1.3 Copper enzymes Enzymes of known structure