|
Kinugasa Reaction
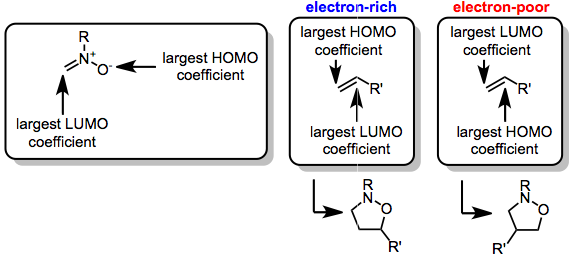
The nitrone-olefin (3+2) cycloaddition reaction is the combination of a nitrone with an alkene or alkyne to generate an isoxazoline or isoxazolidine via a [3+2] cycloaddition process. This reaction is a 1,3-dipolar cycloaddition, in which the nitrone acts as the 1,3-dipole, and the alkene or alkyne as the dipolarophile. Mechanism and stereochemistry When nitrones are combined with either alkenes or alkynes, [3+2] cycloaddition leads to the formation of a new C–C bond and a new C–O bond. The cycloadditions is stereospecific with respect to the configuration of the alkene; however, diastereoselectivity in reactions of C-substituted nitrones is often low. Regioselectivity is controlled by the dominant frontier orbitals interacting during the reaction, and substrates with electronically distinct substituents tend to react with high regioselectivity. Intramolecular versions of the reaction have been used to synthesize complex polyclic carbon frameworks. Reduction of the N–O link ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrone
In organic chemistry, a nitrone is a functional group consisting of an ''N''-oxide of an imine. The general structure is , where R’ is not a hydrogen. A nitrone is a 1,3-dipole, and is used in 1,3-dipolar cycloadditions. Other reactions of nitrones are known, including formal +3cycloadditions to form 6-membered rings, as well as formal +2cycloadditions to form 7-membered rings. Generation of nitrones Nitrones are generated most often either by the oxidation of hydroxylamines or condensation of monosubstituted hydroxylamines with carbonyl compounds (ketones or aldehydes). The most general reagent used for the oxidation of hydroxylamines is mercury(II) oxide. Carbonyl condensation methods avoid issues of site selectivity associated with the oxidation of hydroxylamines with two sets of (alpha) hydrogens. A significant problem associated with many reactive nitrones is dimerization. This issue is alleviated experimentally by employing an excess of the nitrone or increasing t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arkivoc
''Arkivoc'' (''Archive for Organic Chemistry'') is a peer-reviewed open access scientific journal covering all aspects of organic chemistry. It is published by the non-profit organization Arkat USA, which was established in 2000 through a personal donation from Alan R. Katritzky and Linde Katritzky. ''Arkivoc'' is the primary publication of Arkat USA. According to the ''Journal Citation Reports'', the journal has a 2014 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as i ... of 1.165, ranking it 37th out of 57 journals in the category "Chemistry, Organic". Abstracting and Indexing According to the Journal Citation Reports, the journal has a 2018 impact factor of 1.253. The journal is indexed in Web of Science: Science Citation Index Expanded. References External link ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinugasa Reaction
The nitrone-olefin (3+2) cycloaddition reaction is the combination of a nitrone with an alkene or alkyne to generate an isoxazoline or isoxazolidine via a [3+2] cycloaddition process. This reaction is a 1,3-dipolar cycloaddition, in which the nitrone acts as the 1,3-dipole, and the alkene or alkyne as the dipolarophile. Mechanism and stereochemistry When nitrones are combined with either alkenes or alkynes, [3+2] cycloaddition leads to the formation of a new C–C bond and a new C–O bond. The cycloadditions is stereospecific with respect to the configuration of the alkene; however, diastereoselectivity in reactions of C-substituted nitrones is often low. Regioselectivity is controlled by the dominant frontier orbitals interacting during the reaction, and substrates with electronically distinct substituents tend to react with high regioselectivity. Intramolecular versions of the reaction have been used to synthesize complex polyclic carbon frameworks. Reduction of the N–O link ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chem
Chem may refer to: * Chemistry practical waali mam *Chemistry *Chemical * ''Chem'' (journal), a scientific journal published by Cell Press *Post apocalyptic slang for "drugs", medicinal or otherwise in the Fallout video game series. In Ancient Egyptian usage: * ''Khem'' (also spelt ''Chem''), the Egyptian word for "black" * Min (god), in the past erroneously named ''Khem'' CHEM may refer to : *A metabolic panel: for instance, CHEM-7, which is the basic metabolic panel *CHEM-DT CHEM-DT is the TVA owned-and-operated television station in Trois-Rivières, Quebec, Canada. It broadcasts a high-definition digital signal on VHF channel 8 from a transmitter on Rue Principale in Notre-Dame-du-Mont-Carmel. Owned by the Grou ..., a Canadian television channel See also * Chemo (other) * Kemi, a place in Finland {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
β-lactam
A beta-lactam (β-lactam) ring is a four-membered lactam. A ''lactam'' is a cyclic amide, and ''beta''-lactams are named so because the nitrogen atom is attached to the Β carbon, β-carbon atom relative to the carbonyl. The simplest β-lactam possible is 2-azetidinone. β-lactams are significant structural units of medicines as manifested in many Beta-lactam antibiotic, β-lactam antibiotics Up to 1970, most β-lactam research was concerned with the penicillin and cephalosporin groups, but since then, a wide variety of structures have been described. Clinical significance The β-lactam ring is part of the core structure of several antibiotic families, the principal ones being the penicillins, cephalosporins, carbapenems, and monobactams, which are, therefore, also called β-lactam antibiotics. Nearly all of these antibiotics work by inhibiting bacterial cell wall biosynthesis. This has a lethal effect on bacteria, although any given bacteria population will typically contain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetylide
In organometallic chemistry, acetylide refers to chemical compounds with the chemical formulas and , where M is a metal. The term is used loosely and can refer to substituted acetylides having the general structure (where R is an organic side chain). Acetylides are reagents in organic synthesis. The calcium acetylide commonly called calcium carbide is a major compound of commerce. Structure and bonding Alkali metal and alkaline earth metal acetylides of the general formula MC≡CM are salt-like Zintl phase compounds, containing ions. Evidence for this ionic character can be seen in the ready hydrolysis of these compounds to form acetylene and metal oxides, there is also some evidence for the solubility of ions in liquid ammonia. The ion has a closed shell ground state of 1Σ, making it isoelectronic to a neutral molecule N2, which may afford it some stability. Analogous acetylides prepared from other metals, particularly transition metals, show covalent character and are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orange color. Copper is used as a conductor of heat and electricity, as a building material, and as a constituent of various metal alloys, such as sterling silver used in jewelry, cupronickel used to make marine hardware and coins, and constantan used in strain gauges and thermocouples for temperature measurement. Copper is one of the few metals that can occur in nature in a directly usable metallic form ( native metals). This led to very early human use in several regions, from circa 8000 BC. Thousands of years later, it was the first metal to be smelted from sulfide ores, circa 5000 BC; the first metal to be cast into a shape in a mold, c. 4000 BC; and the first metal to be purposely alloyed with another metal, tin, to create ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |