Acetylide on:
[Wikipedia]
[Google]
[Amazon]
In

+ \overset -> ce-78^\circ\ce C] + BuH
Sodium or potassium acetylides can be prepared from various inorganic reagents (such as
organometallic chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
, acetylide refers to chemical compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element ...
s with the chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
s and , where M is a metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typicall ...
. The term is used loosely and can refer to substituted
A substitution reaction (also known as single displacement reaction or single substitution reaction) is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions ar ...
acetylides having the general structure (where R is an organic side chain
In organic chemistry and biochemistry, a side chain is a chemical group that is attached to a core part of the molecule called the "main chain" or backbone. The side chain is a hydrocarbon branching element of a molecule that is attached to a l ...
). Acetylides are reagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
s in organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
. The calcium acetylide commonly called calcium carbide
Calcium carbide, also known as calcium acetylide, is a chemical compound with the chemical formula of Ca C2. Its main use industrially is in the production of acetylene and calcium cyanamide.
The pure material is colorless, while pieces of tec ...
is a major compound of commerce.
Structure and bonding
Alkali metal
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K),The symbols Na and K for sodium and potassium are derived from their Latin names, ''natrium'' and ''kalium''; these are still the origins of the names ...
and alkaline earth metal
The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar properties: they are al ...
acetylides of the general formula MC≡CM are salt-like Zintl phase
In chemistry, a Zintl phase is a product of a reaction between a group 1 (alkali metal) or group 2 (alkaline earth metal) and main group metal or metalloid (from groups 13, 14, 15, or 16). It is characterized by intermediate metallic/ ionic bondin ...
compounds, containing ions. Evidence for this ionic character can be seen in the ready hydrolysis of these compounds to form acetylene
Acetylene (systematic name: ethyne) is the chemical compound with the formula and structure . It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure ...
and metal oxides, there is also some evidence for the solubility of ions in liquid ammonia. The ion has a closed shell
Shell may refer to:
Architecture and design
* Shell (structure), a thin structure
** Concrete shell, a thin shell of concrete, usually with no interior columns or exterior buttresses
** Thin-shell structure
Science Biology
* Seashell, a hard ou ...
ground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. ...
of 1Σ, making it isoelectronic
Isoelectronicity is a phenomenon observed when two or more molecules have the same structure (positions and connectivities among atoms) and the same electronic configurations, but differ by what specific elements are at certain locations in the ...
to a neutral molecule N2, which may afford it some stability.
Analogous acetylides prepared from other metals, particularly transition metals
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
, show covalent character and are invariably associated with their metal centers. This can be seen in their general stability to water (such as silver acetylide
Silver acetylide is an inorganic chemical compound with the formula Ag2C2, a metal acetylide. The compound can be regarded as a salt of the weak acid, acetylene. The salt's anion consists of two carbon atoms linked by a triple bond. The alternate ...
, copper acetylide
Copper(I) acetylide, or cuprous acetylide, is a chemical compound with the formula Cu2 C2. Although never characterized by X-ray crystallography, the material has been claimed at least since 1856. One form is claimed to be a monohydrate with for ...
) and radically different chemical applications.
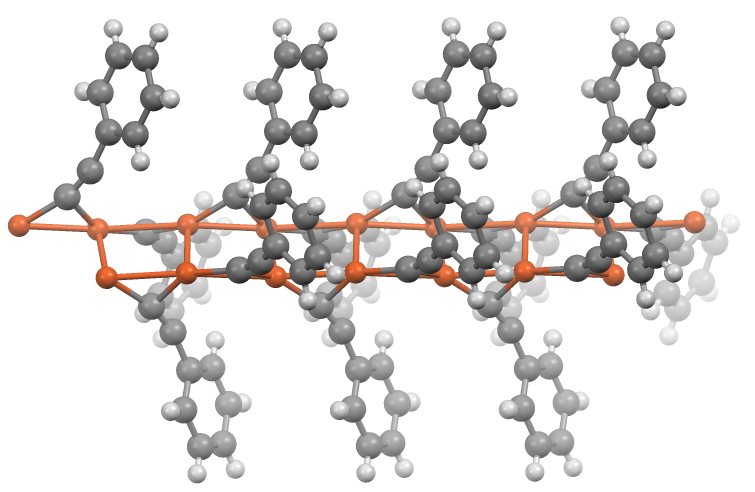
Acetylides of the general formula RC≡CM (where R = H or alkyl) generally show similar properties to their doubly substituted analogues. In the absence of additional ligands, metal acetylides adopt polymeric structures wherein the acetylide groups are bridging ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually r ...
s.

Preparation
Terminalalkyne
\ce
\ce
Acetylene
\ce
\ce
\ce
Propyne
\ce
\ce
\ce
\ce
1-Butyne
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...
s are weak acids
Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a proton, H+, and an anion, A-. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solution ...
:
: RC≡CH + R″M R″H + RC≡CM
To generate acetylides from acetylene and alkynes relies on the use of organometallic
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so ...
or inorganic
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemist ...
superbase
A superbase is a compound that has a particularly high affinity for protons. Superbases are of theoretical interest and potentially valuable in organic synthesis. Superbases have been described and used since the 1850s.''Superbases for Organic ...
s in solvents which are less acidic than the terminal alkyne. In early studies liquid ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous wast ...
was employed, but ethereal solvents are more common.
Lithium amide
Lithium amide or lithium azanide is an inorganic compound with the chemical formula . It is a white solid with a tetragonal crystal structure. Lithium amide can be made by treating lithium metal with liquid ammonia:
:
Other lithium amides
The co ...
, LiHMDS
Lithium bis(trimethylsilyl)amide is a lithiated organosilicon compound with the formula . It is commonly abbreviated as LiHMDS or Li(HMDS) (lithium hexamethyldisilazide - a reference to its conjugate acid HMDS) and is primarily used as a strong ...
, or organolithium reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
s, such as butyllithium Butyllithium may refer to one of 5 isomeric organolithium reagents of which 3 are commonly used in chemical synthesis:
* ''n''-Butyllithium, abbreviated BuLi or nBuLi
* ''sec''-Butyllithium, abbreviated ''sec''-BuLi or sBuLi, has 2 stereoisomers, ...
, are frequently used to form lithium acetylides:
:sodium amide
Sodium amide, commonly called sodamide (systematic name sodium azanide), is the inorganic compound with the formula . It is a salt composed of the sodium cation and the azanide anion. This solid, which is dangerously reactive toward water, is whit ...
) or from their elemental metals, often at room temperature and atmospheric pressure.
Copper(I) acetylide
Copper(I) acetylide, or cuprous acetylide, is a chemical compound with the formula copper, Cu2carbon, C2. Although never characterized by X-ray crystallography, the material has been claimed at least since 1856. One form is claimed to be a monohy ...
can be prepared by passing acetylene through an aqueous
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, or sodium chloride (NaCl), in water would be rep ...
solution of copper(I) chloride
Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear gre ...
because of a low solubility equilibrium Solubility equilibrium is a type of dynamic equilibrium that exists when a chemical compound in the solid state is in chemical equilibrium with a solution of that compound. The solid may dissolve unchanged, with dissociation, or with chemical reacti ...
. Similarly, silver acetylide
Silver acetylide is an inorganic chemical compound with the formula Ag2C2, a metal acetylide. The compound can be regarded as a salt of the weak acid, acetylene. The salt's anion consists of two carbon atoms linked by a triple bond. The alternate ...
s can be obtained from silver nitrate
Silver nitrate is an inorganic compound with chemical formula . It is a versatile precursor to many other silver compounds, such as those used in photography. It is far less sensitive to light than the halides. It was once called ''lunar caustic' ...
.
Calcium carbide
Calcium carbide, also known as calcium acetylide, is a chemical compound with the chemical formula of Ca C2. Its main use industrially is in the production of acetylene and calcium cyanamide.
The pure material is colorless, while pieces of tec ...
is prepared by heating carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with o ...
with lime
Lime commonly refers to:
* Lime (fruit), a green citrus fruit
* Lime (material), inorganic materials containing calcium, usually calcium oxide or calcium hydroxide
* Lime (color), a color between yellow and green
Lime may also refer to:
Botany ...
(calcium oxide
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, Caustic (substance), caustic, alkaline, crystalline solid at room temperature. The broadly used term "''lime (material), lime''" co ...
) at approximately 2,000 °C. A similar process is used to produce lithium carbide
Lithium carbide, , often known as dilithium acetylide, is a chemical compound of lithium and carbon, an acetylide. It is an intermediate compound produced during radiocarbon dating procedures. is one of an extensive range of lithium-carbon compo ...
.
Reactions
Acetylides of the type RC2M are widely used inalkynylation
In organic chemistry, alkynylation is an addition reaction in which a terminal alkyne () is added to a carbonyl group () to form an Alpha and beta carbon, α-alkynyl alcohol (chemistry), alcohol ().
When the acetylide is formed from acetylene () ...
s in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
. They are nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
s that add to a variety of electrophilic and unsaturated substrates. A classic application is the Favorskii reaction
The Favorskii reaction is an organic chemistry reaction between an alkyne and a carbonyl group, under base (chemistry), basic conditions. The reaction was discovered in the early 1900s by the Russian chemist Alexei Yevgrafovich Favorskii.
When ...
.
Illustrative is the sequence shown below, ethyl propiolate
Ethyl propiolate is an organic compound with the formula HC2CO2C2H5. It is the ethyl ester of propiolic acid, the simplest acetylenic carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl grou ...
is deprotonated by ''n''-butyllithium to give the corresponding acetylide. This acetylide adds to the carbonyl center of cyclopentanone
Cyclopentanone is the organic compound with the formula (CH2)4CO. This cyclic ketone is a colorless volatile liquid.
Preparation
Upon treatment with barium hydroxide at elevated temperatures, adipic acid undergoes ketonization to give cyclopenta ...
. Hydrolytic workup liberate the alkynyl alcohol.
Coupling reactions
Acetylides are sometimes intermediates incoupling reaction A coupling reaction in organic chemistry is a general term for a variety of reactions where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = ...
s. Examples include Sonogashira coupling
The Sonogashira reaction is a cross-coupling reaction used in organic synthesis to form carbon–carbon bonds. It employs a palladium catalyst as well as copper co-catalyst to form a carbon–carbon bond between a terminal alkyne and an aryl or vi ...
, Cadiot-Chodkiewicz coupling, Glaser coupling
The Glaser coupling is a type of coupling reaction. It is by far the oldest acetylenic coupling and is based on cuprous salts like copper(I) chloride or copper(I) bromide and an additional oxidant like oxygen. The base in its original scope is ammo ...
and Eglinton coupling
The Glaser coupling is a type of coupling reaction. It is by far the oldest acetylenic coupling and is based on cuprous salts like copper(I) chloride or copper(I) bromide and an additional oxidant like oxygen. The base in its original scope is ammo ...
.
Hazards
Some acetylides are notoriously explosive. Formation of acetylides poses a risk in handling of gaseous acetylene in presence of metals such asmercury
Mercury commonly refers to:
* Mercury (planet), the nearest planet to the Sun
* Mercury (element), a metallic chemical element with the symbol Hg
* Mercury (mythology), a Roman god
Mercury or The Mercury may also refer to:
Companies
* Merc ...
, silver
Silver is a chemical element with the Symbol (chemistry), symbol Ag (from the Latin ', derived from the Proto-Indo-European wikt:Reconstruction:Proto-Indo-European/h₂erǵ-, ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, whi ...
or copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkis ...
, or alloys with their high content (brass
Brass is an alloy of copper (Cu) and zinc (Zn), in proportions which can be varied to achieve different mechanical, electrical, and chemical properties. It is a substitutional alloy: atoms of the two constituents may replace each other with ...
, bronze
Bronze is an alloy consisting primarily of copper, commonly with about 12–12.5% tin and often with the addition of other metals (including aluminium, manganese, nickel, or zinc) and sometimes non-metals, such as phosphorus, or metalloids such ...
, silver solder
Solder (; NA: ) is a fusible metal alloy used to create a permanent bond between metal workpieces. Solder is melted in order to wet the parts of the joint, where it adheres to and connects the pieces after cooling. Metals or alloys suitable ...
).
See also
*Ethynyl In organic chemistry, the term ethynyl designates a functional group with a double bond with 2 carbon atoms both with
sp hybridisation and a triple bond(1 sigma and 2 pi bond) . It is a species similar to acetylene (or in IUPAC ethyne ) with a less ...
* Ethynyl radical
The ethynyl radical (systematically named λ3-ethyne and hydridodicarbon(''C''—''C'')) is an organic compound with the chemical formula C≡CH (also written CHor ). It is a simple molecule that does not occur naturally on Earth but is abundant ...
* Diatomic carbon
Diatomic carbon (systematically named dicarbon and 1λ2,2λ2-ethene), is a green, gaseous inorganic chemical with the chemical formula C=C (also written 2or C2). It is kinetically unstable at ambient temperature and pressure, being removed throug ...
(neutral C2)
* Acetylenediol
Acetylenediol, or ethynediol, is a chemical substance with formula HO−C≡C−OH (an ynol). It is the diol of acetylene. Acetylenediol is unstable in the condensed phase, although its tautomer glyoxal (CHO)2 is well known.
Detection
Acetyl ...
References
{{reflist Anions Functional groups *