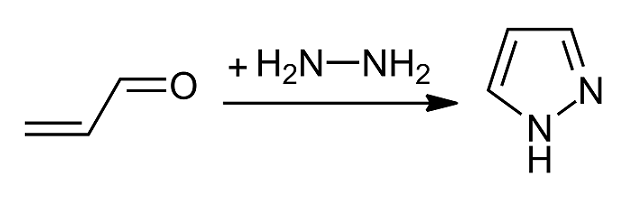|
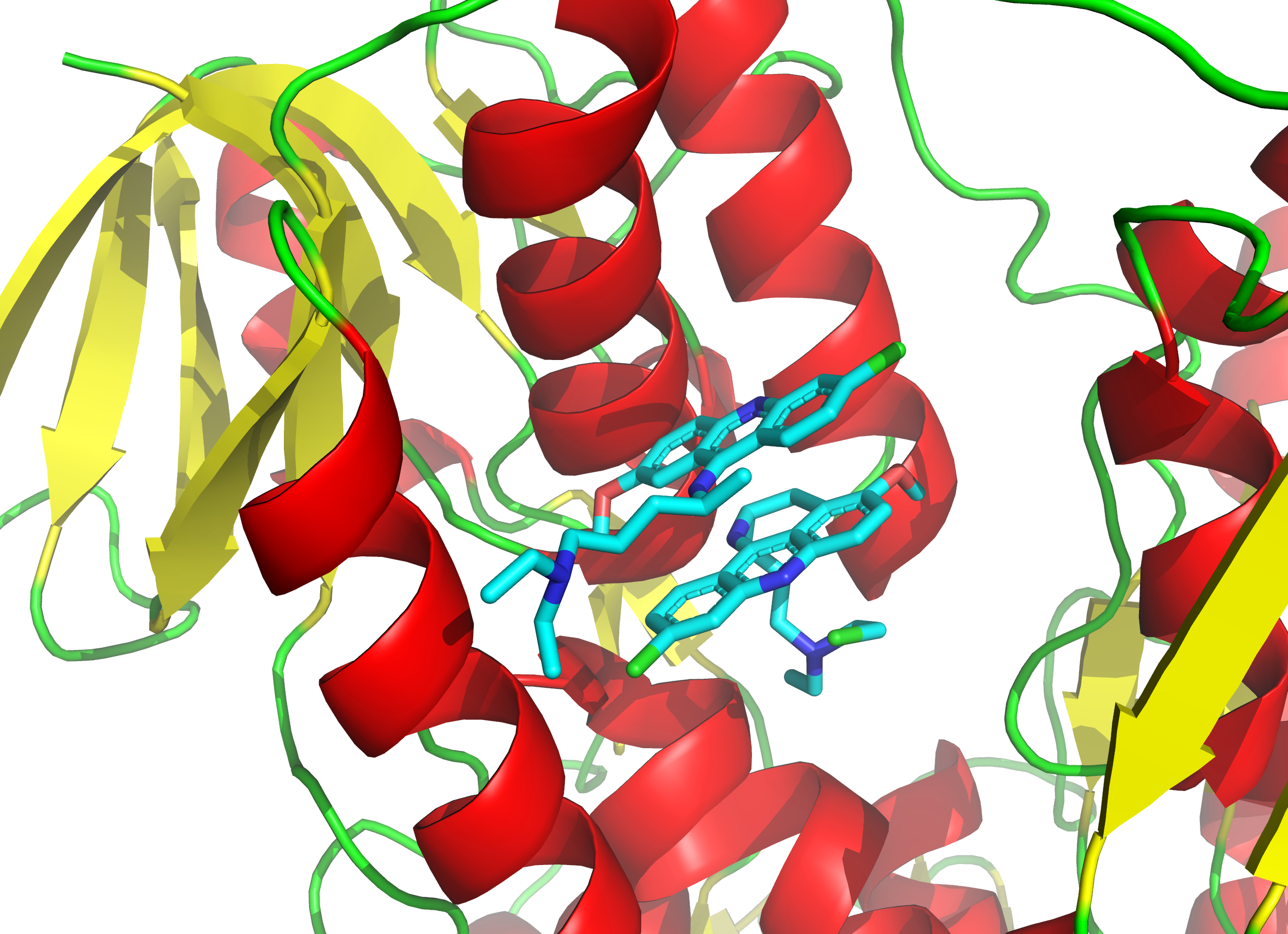
IMP-1088
IMP-1088 is an enzyme inhibitor of the human N-myristoyltransferases NMT1 and NMT2 capable of preventing rhinoviral replication, an area of research relating to potential treatment of the common cold The common cold or the cold is a viral infectious disease of the upper respiratory tract that primarily affects the respiratory mucosa of the nose, throat, sinuses, and larynx. Signs and symptoms may appear fewer than two days after exposu .... IMP-1088 works to keep cells from generating infectious virus by targeting the cell instead of the rhinovirus itself. It does this by blocking the NMT protein of the host cell which prevents the virus from assembling its capsid, since viral capsid myristoylation by host NMT is essential for assembly. It is thought unlikely that viruses will evolve resistance to such an approach since IMP-1088 works against the human cell and not the virus. References {{reflist Enzyme inhibitors Indazoles Pyrazoles Fluoroarenes Biphenyls Dime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhinovirus
The rhinovirus (from the grc, ῥίς, rhis "nose", , romanized: "of the nose", and the la, vīrus) is the most common viral infectious agent in humans and is the predominant cause of the common cold. Rhinovirus infection proliferates in temperatures of 33–35 °C (91–95 °F), the temperatures found in the nose. Rhinoviruses belong to the genus ''Enterovirus'' in the family ''Picornaviridae''. The three species of rhinovirus (A, B, and C) include around 160 recognized types (called serotypes) of human rhinovirus that differ according to their surface antigens. They are lytic in nature and are among the smallest viruses, with diameters of about 30 nanometers. By comparison, other viruses, such as smallpox and vaccinia, are around ten times larger at about 300 nanometers, while flu viruses are around 80–120 nm. History In 1953, when a cluster of nurses developed a mild respiratory illness, Winston Price, from the Johns Hopkins University, took nasal pas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme Inhibitor
An enzyme inhibitor is a molecule that binds to an enzyme and blocks its activity. Enzymes are proteins that speed up chemical reactions necessary for life, in which substrate molecules are converted into products. An enzyme facilitates a specific chemical reaction by binding the substrate to its active site, a specialized area on the enzyme that accelerates the most difficult step of the reaction. An enzyme inhibitor stops ("inhibits") this process, either by binding to the enzyme's active site (thus preventing the substrate itself from binding) or by binding to another site on the enzyme such that the enzyme's catalysis of the reaction is blocked. Enzyme inhibitors may bind reversibly or irreversibly. Irreversible inhibitors form a chemical bond with the enzyme such that the enzyme is inhibited until the chemical bond is broken. By contrast, reversible inhibitors bind non-covalently and may spontaneously leave the enzyme, allowing the enzyme to resume its function. Reve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-myristoyltransferase 1
Glycylpeptide N-tetradecanoyltransferase 1 also known as myristoyl-CoA:protein N-myristoyltransferase 1 (NMT-1) is an enzyme that in humans is encoded by the ''NMT1'' gene. It belongs to the protein N-terminal methyltransferase and glycylpeptide N-tetradecanoyltransferase family of enzymes. References Further reading * * * * * * * * * * * * * * * * * * * See also * Myristoylation * NMT2, N-myristoyltransferase 2 External links * EC 2.3.1 Human proteins {{gene-17-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NMT2
Glycylpeptide N-tetradecanoyltransferase 2 known also as N-myristoyltransferase, is an enzyme (EC: 2.3.1.97) that in humans is encoded by the ''NMT2'' gene. Function N-myristoyltransferase (NMT) catalyzes the reaction of N-terminal myristoylation of many signaling proteins. It transfers myristic acid from myristoyl coenzyme A to the amino group of a protein's N-terminal glycine residue. Biochemical evidence indicates the presence of several distinct NMTs, varying in apparent molecular weight and /or subcellular distribution. The 496-amino acid of human NMT2 protein shares 77% and 96% sequence identity with human NMT1 and mouse Nmt2 comprise two distinct families of N-myristoyltransferases. Interactions NMT2 has been shown to interact with: * caspase 3 * MARCKS See also * N-myristoyltransferase 1 Glycylpeptide N-tetradecanoyltransferase 1 also known as myristoyl-CoA:protein N-myristoyltransferase 1 (NMT-1) is an enzyme that in humans is encoded by the ''NMT1'' gene. It be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Common Cold
The common cold or the cold is a viral infectious disease of the upper respiratory tract that primarily affects the respiratory mucosa of the nose, throat, sinuses, and larynx. Signs and symptoms may appear fewer than two days after exposure to the virus. These may include coughing, sore throat, runny nose, sneezing, headache, and fever. People usually recover in seven to ten days, but some symptoms may last up to three weeks. Occasionally, those with other health problems may develop pneumonia. Well over 200 virus strains are implicated in causing the common cold, with rhinoviruses, coronaviruses, adenoviruses and enteroviruses being the most common. They spread through the air during close contact with infected people or indirectly through contact with objects in the environment, followed by transfer to the mouth or nose. Risk factors include going to child care facilities, not sleeping well, and psychological stress. The symptoms are mostly due to the body's immune ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capsid
A capsid is the protein shell of a virus, enclosing its genetic material. It consists of several oligomeric (repeating) structural subunits made of protein called protomers. The observable 3-dimensional morphological subunits, which may or may not correspond to individual proteins, are called capsomeres. The proteins making up the capsid are called capsid proteins or viral coat proteins (VCP). The capsid and inner genome is called the nucleocapsid. Capsids are broadly classified according to their structure. The majority of the viruses have capsids with either helical or icosahedral structure. Some viruses, such as bacteriophages, have developed more complicated structures due to constraints of elasticity and electrostatics. The icosahedral shape, which has 20 equilateral triangular faces, approximates a sphere, while the helical shape resembles the shape of a spring, taking the space of a cylinder but not being a cylinder itself. The capsid faces may consist of one or more ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme Inhibitors
An enzyme inhibitor is a molecule that binds to an enzyme and blocks its activity. Enzymes are proteins that speed up chemical reactions necessary for life, in which substrate molecules are converted into products. An enzyme facilitates a specific chemical reaction by binding the substrate to its active site, a specialized area on the enzyme that accelerates the most difficult step of the reaction. An enzyme inhibitor stops ("inhibits") this process, either by binding to the enzyme's active site (thus preventing the substrate itself from binding) or by binding to another site on the enzyme such that the enzyme's catalysis of the reaction is blocked. Enzyme inhibitors may bind reversibly or irreversibly. Irreversible inhibitors form a chemical bond with the enzyme such that the enzyme is inhibited until the chemical bond is broken. By contrast, reversible inhibitors bind non-covalently and may spontaneously leave the enzyme, allowing the enzyme to resume its function. Reve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indazoles
Indazole, also called isoindazole, is a heterocyclic aromatic organic compound. This bicyclic compound consists of the fusion of benzene and pyrazole. Indazole is an Amphoterism, amphoteric molecule which can be protonated to an indazolium cation or deprotonated to an indazolate anion. The corresponding ''pKa'' values are 1.04 for the equilibrium between indazolium cation and indazole and 13.86 for the equilibrium between indazole and indazolate anion. Indazole derivatives display a broad variety of biological activities. Indazoles are rare in nature. The alkaloids nigellicine, nigeglanine, and nigellidine are indazoles. Nigellicine was isolated from the widely distributed plant ''Nigella sativa'' L. (black cumin). Nigeglanine was isolated from extracts of ''Nigella glandulifera''. The Davis–Beirut reaction can generate 2''H''-indazoles. Indazole, C7H6N2, was obtained by E. Fischer (''Ann.'' 1883, 221, p. 280) by heating ortho-hydrazine cinnamic acid, : Some derivatives ; i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrazoles
Pyrazole is an organic compound with the formula C3H3N2H. It is a heterocycle characterized by a 5-membered ring of three carbon atoms and two adjacent nitrogen atoms, which are in ortho-substitution. Pyrazole is a weak base, with p''K''b 11.5 (p''K''a of the conjugate acid 2.49 at 25 °C). Pyrazoles are also a class of compounds that have the ring C3N2 with adjacent nitrogen atoms. Notable drugs containing a pyrazole ring are celecoxib (celebrex) and the anabolic steroid stanozolol. Preparation and reactions Pyrazoles are synthesized by the reaction of α,β-unsaturated aldehydes with hydrazine and subsequent dehydrogenation: : Substituted pyrazoles are prepared by condensation of 1,3-diketones with hydrazine ( Knorr-type reactions). For example, acetylacetone and hydrazine gives 3,5-dimethylpyrazole: :CH3C(O)CH2C(O)CH3 + N2H4 → (CH3)2C3HN2H + 2 H2O History The term pyrazole was given to this class of compounds by German ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.gif)