|
Electrolytic Capacitor
An electrolytic capacitor is a polarized capacitor whose anode or positive plate is made of a metal that forms an insulating oxide layer through anodization. This oxide layer acts as the dielectric of the capacitor. A solid, liquid, or gel electrolyte covers the surface of this oxide layer, serving as the cathode or negative plate of the capacitor. Due to their very thin dielectric oxide layer and enlarged anode surface, electrolytic capacitors have a much higher capacitance-voltage (CV) product per unit volume than ceramic capacitors or film capacitors, and so can have large capacitance values. There are three families of electrolytic capacitor: aluminum electrolytic capacitors, tantalum electrolytic capacitors, and niobium electrolytic capacitors. The large capacitance of electrolytic capacitors makes them particularly suitable for passing or bypassing low-frequency signals, and for storing large amounts of energy. They are widely used for decoupling or noise filtering ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminum Electrolytic Capacitor
Aluminum electrolytic capacitors are polarized electrolytic capacitors whose anode electrode (+) is made of a pure aluminum foil with an etched surface. The aluminum forms a very thin insulating layer of aluminum oxide by anodization that acts as the dielectric of the capacitor. A non-solid electrolyte covers the rough surface of the oxide layer, serving in principle as the second electrode (cathode) (-) of the capacitor. A second aluminum foil called “cathode foil” contacts the electrolyte and serves as the electrical connection to the negative terminal of the capacitor. Aluminum electrolytic capacitors are divided into three subfamilies by electrolyte type: *non-solid (liquid, wet) aluminum electrolytic capacitors, * solid manganese dioxide aluminum electrolytic capacitors, and * solid polymer aluminum electrolytic capacitors. Aluminum electrolytic capacitors with non-solid electrolyte are the most inexpensive type and also those with widest range of sizes, capacitance ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
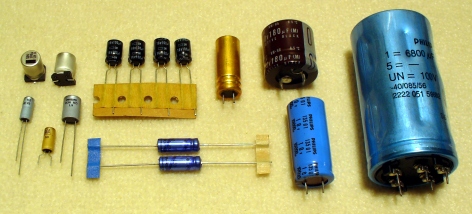
Electrolytic Capacitors-P1090328
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved. Electrically, such a solution is neutral. If an electric potential is applied to such a solution, the cations of the solution are drawn to the electrode that has an abundance of electrons, while the anions are drawn to the electrode that has a deficit of electrons. The movement of anions and cations in opposite directions within the solution amounts to a current. Some gases, such as hydrogen chloride (HCl), under conditions of high temperature or low pressure can also function as electrolytes. Ele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronic Filter
Electronic filters are a type of signal processing filter in the form of electrical circuits. This article covers those filters consisting of lumped electronic components, as opposed to distributed-element filters. That is, using components and interconnections that, in analysis, can be considered to exist at a single point. These components can be in discrete packages or part of an integrated circuit. Electronic filters remove unwanted frequency components from the applied signal, enhance wanted ones, or both. They can be: *passive or active *analog or digital *high-pass, low-pass, band-pass, band-stop (band-rejection; notch), or all-pass. *discrete-time (sampled) or continuous-time *linear or non-linear *infinite impulse response (IIR type) or finite impulse response (FIR type) The most common types of electronic filters are linear filters, regardless of other aspects of their design. See the article on linear filters for details on their design and analysis. Histo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
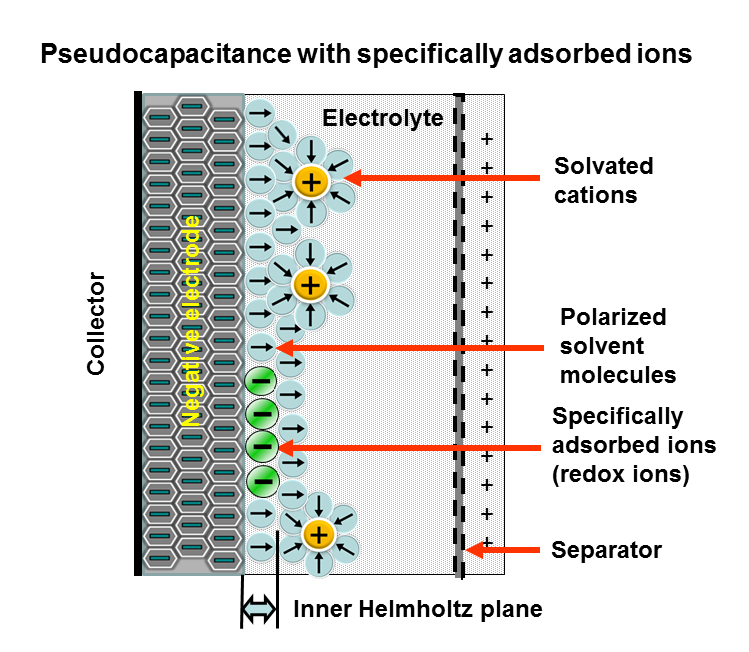
Pseudocapacitance
Pseudocapacitance is the electrochemical storage of electricity in an electrochemical capacitor (Pseudocapacitor). This faradaic charge transfer originates by a very fast sequence of reversible faradaic redox, electrosorption or intercalation processes on the surface of suitable electrodes. see alsBrian E. Conway in Electrochemistry Encyclopedia: ''ELECTROCHEMICAL CAPACITORS Their Nature, Function, and Applications''E. Frackowiak, F. Beguin: ''Carbon Materials For The Electrochemical Storage Of Energy In Capacitors.'' In: ''CARBON.'' 39, 2001, S. 937–950PDF E. Frackowiak, K. Jurewicz, S. Delpeux, F. Béguin: ''Nanotubular Materials For Supercapacitors.'' In: ''Journal of Power Sources.'' Volumes 97–98, Juli 2001, S. 822–825, . Pseudocapacitance is accompanied by an electron charge-transfer between electrolyte and electrode coming from a de-solvated and adsorbed ion. One electron per charge unit is involved. The adsorbed ion has no chemical reaction with the atoms of the e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double-layer Capacitance
Double-layer capacitance is the important characteristic of the electrical double layer which appears, for example, at the interface between a conductive electrode and an adjacent liquid electrolyte. At this boundary two layers of charge with opposing polarity form, one at the surface of the electrode, and one in the electrolyte. These two layers, electrons on the electrode and ions in the electrolyte, are typically separated by a single layer of solvent molecules that adhere to the surface of the electrode and act like a dielectric in a conventional capacitor. The amount of electric charge stored in double-layer capacitor depends on the applied voltage. The unit of capacitance is the farad. The double-layer capacitance is the physical principle behind the electrostatic double-layer type of Supercapacitors. History * Development of the double layer and pseudocapacitance model see Double layer (interfacial) * Development of the electrochemical components see Supercapacitors Cap ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supercapacitor
A supercapacitor (SC), also called an ultracapacitor, is a high-capacity capacitor, with a capacitance value much higher than other capacitors but with lower voltage limits. It bridges the gap between electrolytic capacitors and rechargeable batteries. It typically stores 10 to 100 times more energy per unit volume or mass than electrolytic capacitors, can accept and deliver charge much faster than batteries, and tolerates many more charge and discharge cycles than rechargeable batteries. Supercapacitors are used in applications requiring many rapid charge/discharge cycles, rather than long-term compact energy storage — in automobiles, buses, trains, cranes and elevators, where they are used for regenerative braking, short-term energy storage, or burst-mode power delivery. Smaller units are used as power backup for static random-access memory (SRAM). Unlike ordinary capacitors, supercapacitors do not use the conventional solid dielectric, but rather, they use electrosta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials depending on the type of battery. The electrophore, invented by Johan Wilcke, was an early version of an electrode used to study static electricity. Anode and cathode in electrochemical cells Electrodes are an essential part of any battery. The first electrochemical battery made was devised by Alessandro Volta and was aptly named the Voltaic cell. This battery consisted of a stack of copper and zinc electrodes separated by brine-soaked paper disks. Due to fluctuation in the voltage provided by the voltaic cell it wasn't very practical. The first practical battery was invented in 1839 and named the Daniell cell after John Frederic Daniell. Still making use of the zinc–copper electrode combination. Since then many more batteries have be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Field
An electric field (sometimes E-field) is the physical field that surrounds electrically charged particles and exerts force on all other charged particles in the field, either attracting or repelling them. It also refers to the physical field for a system of charged particles. Electric fields originate from electric charges and time-varying electric currents. Electric fields and magnetic fields are both manifestations of the electromagnetic field, one of the four fundamental interactions (also called forces) of nature. Electric fields are important in many areas of physics, and are exploited in electrical technology. In atomic physics and chemistry, for instance, the electric field is the attractive force holding the atomic nucleus and electrons together in atoms. It is also the force responsible for chemical bonding between atoms that result in molecules. The electric field is defined as a vector field that associates to each point in space the electrostatic ( Coulomb) for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Charge
Electric charge is the physical property of matter that causes charged matter to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative'' (commonly carried by protons and electrons respectively). Like charges repel each other and unlike charges attract each other. An object with an absence of net charge is referred to as neutral. Early knowledge of how charged substances interact is now called classical electrodynamics, and is still accurate for problems that do not require consideration of quantum effects. Electric charge is a conserved property; the net charge of an isolated system, the amount of positive charge minus the amount of negative charge, cannot change. Electric charge is carried by subatomic particles. In ordinary matter, negative charge is carried by electrons, and positive charge is carried by the protons in the nuclei of atoms. If there are more electrons than protons in a piece of matter, it will have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Static Electricity
Static electricity is an imbalance of electric charges within or on the surface of a material or between materials. The charge remains until it is able to move away by means of an electric current or electrical discharge. Static electricity is named in contrast with current electricity, where the electric charge flows through an electrical conductor or space, and transmits energy. A static electric charge can be created whenever two surfaces contact and have worn and separated, and at least one of the surfaces has a high resistance to electric current (and is therefore an electrical insulator). The effects of static electricity are familiar to most people because people can feel, hear, and even see the spark as the excess charge is neutralized when brought close to a large electrical conductor (for example, a path to ground), or a region with an excess charge of the opposite polarity (positive or negative). The familiar phenomenon of a static shockmore specifically, an electros ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Energy
Electrical energy is energy related to forces on electrically charged particles and the movement of electrically charged particles (often electrons in wires, but not always). This energy is supplied by the combination of electric current and electric potential (often referred to as voltage because electric potential is measured in volts) that is delivered by an electrical circuit (e.g., provided by an electric power utility). Motion (current) is not required; for example, if there is a voltage difference in combination with charged particles, such as static electricity or a charged capacitor, the moving electrical energy is typically converted to another form of energy (e.g., thermal, motion, sound, light, radio waves, etc.). Electrical energy is usually sold by the kilowatt hour (1 kW·h = 3.6 MJ) which is the product of the power in kilowatts multiplied by running time in hours. Electric utilities measure energy using an electricity meter, which keeps a running total of the elec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |





.jpg)