|
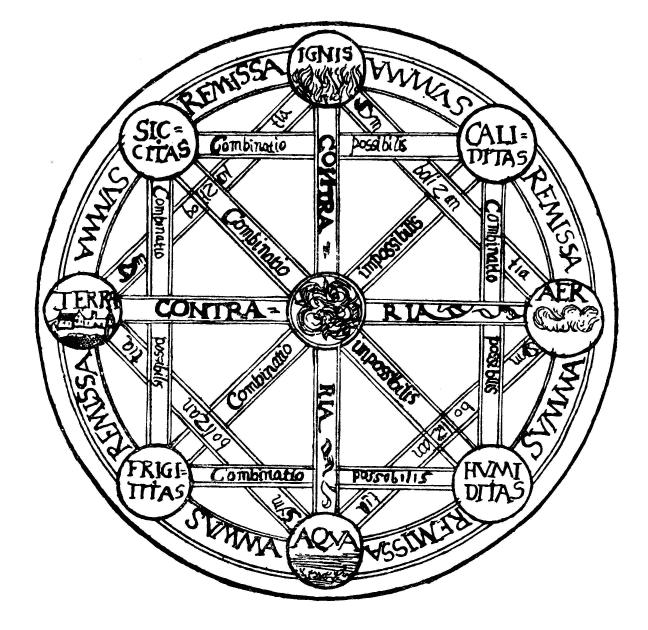
Classical Element
The classical elements typically refer to Earth (classical element), earth, Water (classical element), water, Air (classical element), air, Fire (classical element), fire, and (later) Aether (classical element), aether which were proposed to explain the nature and complexity of all matter in terms of simpler Substance theory, substances. Ancient cultures in Ancient Greece, Greece, Angola, Ancient Tibet, Tibet, Ancient India, India, and Mali had similar lists which sometimes referred, in local languages, to "air" as "wind", and to "aether" as "space". These different cultures and even individual philosophers had widely varying explanations concerning their attributes and how they related to observable phenomena as well as cosmology. Sometimes these theories overlapped with mythology and were personification, personified in deities. Some of these interpretations included atomism (the idea of very small, indivisible portions of matter), but other interpretations considered the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Leibniz Four Elements
Gottfried Wilhelm Leibniz (or Leibnitz; – 14 November 1716) was a German polymath active as a mathematician, philosopher, scientist and diplomat who is credited, alongside Isaac Newton, Sir Isaac Newton, with the creation of calculus in addition to many other branches of mathematics, such as binary arithmetic and statistics. Leibniz has been called the "last universal genius" due to his vast expertise across fields, which became a rarity after his lifetime with the coming of the Industrial Revolution and the spread of specialized labor. He is a prominent figure in both the history of philosophy and the history of mathematics. He wrote works on philosophy, theology, ethics, politics, law, history, philology, games, music, and other studies. Leibniz also made major contributions to physics and technology, and anticipated notions that surfaced much later in probability theory, biology, medicine, geology, psychology, linguistics and computer science. Leibniz contributed to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Personification
Personification is the representation of a thing or abstraction as a person, often as an embodiment or incarnation. In the arts, many things are commonly personified, including: places, especially cities, National personification, countries, and continents; elements of the natural world, such as trees, the Deities and personifications of seasons, four seasons, the "four elements", the Anemoi, four cardinal winds, and the Sense, five senses; moral abstractions, especially the four cardinal virtues and seven deadly sins; the nine Muses; and Personifications of death, death. In many polytheistic early religions, deity, deities had a strong element of personification, suggested by descriptions such as "god of". In ancient Greek religion, and the related ancient Roman religion, this was perhaps especially strong, in particular among the minor deities. Many such deities, such as the or tutelary deities for major cities, survived the arrival of Christianity, now as symbolic personif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Chemical Element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its atomic nucleus, nucleus. Atoms of the same element can have different numbers of neutrons in their nuclei, known as isotopes of the element. Two or more atoms can combine to form molecules. Some elements form Homonuclear molecule, molecules of atoms of said element only: e.g. atoms of hydrogen (H) form Diatomic molecule, diatomic molecules (H). Chemical compounds are substances made of atoms of different elements; they can have molecular or non-molecular structure. Mixtures are materials containing different chemical substances; that means (in case of molecular substances) that they contain different types of molecules. Atoms of one element can be transformed into atoms of a different element in nuclear reactions, which change an atom's at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Atomic Theory
Atomic theory is the scientific theory that matter is composed of particles called atoms. The definition of the word "atom" has changed over the years in response to scientific discoveries. Initially, it referred to a hypothetical concept of there being some fundamental particle of matter, too small to be seen by the naked eye, that could not be divided. Then the definition was refined to being the basic particles of the chemical elements, when chemists observed that elements seemed to combine with each other in ratios of small whole numbers. Then physicists discovered that these particles had an internal structure of their own and therefore perhaps did not deserve to be called "atoms", but renaming atoms would have been impractical by that point. Atomic theory is one of the most important scientific developments in history, crucial to all the physical sciences. At the start of ''The Feynman Lectures on Physics'', physicist and Nobel laureate Richard Feynman offers the atomic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
History Of Science
The history of science covers the development of science from ancient history, ancient times to the present. It encompasses all three major branches of science: natural science, natural, social science, social, and formal science, formal. Protoscience, Science in the ancient world, early sciences, and natural philosophies such as alchemy and astrology that existed during the Bronze Age, Iron Age, classical antiquity and the Middle Ages, declined during the early modern period after the establishment of formal disciplines of science in the Age of Enlightenment. The earliest roots of scientific thinking and practice can be traced to Ancient Egypt and Mesopotamia during the 3rd and 2nd millennia BCE. These civilizations' contributions to mathematics, astronomy, and medicine influenced later Greek natural philosophy of Science in classical antiquity, classical antiquity, wherein formal attempts were made to provide explanations of events in the Universe, physical world based on n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Scientific Revolution
The Scientific Revolution was a series of events that marked the emergence of History of science, modern science during the early modern period, when developments in History of mathematics#Mathematics during the Scientific Revolution, mathematics, History of physics#Scientific Revolution, physics, History of astronomy#Renaissance Period, astronomy, History of biology#Renaissance and early modern developments, biology (including History of anatomy, human anatomy) and History of chemistry#17th and 18th centuries: Early chemistry, chemistry transformed the views of society about nature.Galilei, Galileo (1974) ''Two New Sciences'', trans. Stillman Drake, (Madison: Univ. of Wisconsin Pr. pp. 217, 225, 296–67.Clagett, Marshall (1961) ''The Science of Mechanics in the Middle Ages''. Madison, Univ. of Wisconsin Pr. pp. 218–19, 252–55, 346, 409–16, 547, 576–78, 673–82#Hannam, Hannam, p. 342 The Scientific Revolution took place in Europe in the second half of the Renaissance pe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hippocrates
Hippocrates of Kos (; ; ), also known as Hippocrates II, was a Greek physician and philosopher of the Classical Greece, classical period who is considered one of the most outstanding figures in the history of medicine. He is traditionally referred to as the "Father of Medicine" in recognition of his lasting contributions to the field, such as the use of prognosis and clinical observation, the systematic categorization of diseases, and the (however misguided) formulation of Humorism, humoral theory. His studies set out the basic ideas of modern-day specialties, including surgery, urology, neurology, acute medicine and Orthopedic surgery, orthopedics. The Hippocratic school of medicine revolutionized ancient Greek medicine, establishing it as a discipline distinct from other fields with which it had traditionally been associated (theurgy and philosophy), thus establishing medicine as a profession. However, the achievements of the writers of the Hippocratic Corpus, the practitioners ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Aristotle
Aristotle (; 384–322 BC) was an Ancient Greek philosophy, Ancient Greek philosopher and polymath. His writings cover a broad range of subjects spanning the natural sciences, philosophy, linguistics, economics, politics, psychology, and the arts. As the founder of the Peripatetic school of philosophy in the Lyceum (classical), Lyceum in Athens, he began the wider Aristotelianism, Aristotelian tradition that followed, which set the groundwork for the development of modern science. Little is known about Aristotle's life. He was born in the city of Stagira (ancient city), Stagira in northern Greece during the Classical Greece, Classical period. His father, Nicomachus (father of Aristotle), Nicomachus, died when Aristotle was a child, and he was brought up by a guardian. At around eighteen years old, he joined Plato's Platonic Academy, Academy in Athens and remained there until the age of thirty seven (). Shortly after Plato died, Aristotle left Athens and, at the request ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Empedocles
Empedocles (; ; , 444–443 BC) was a Ancient Greece, Greek pre-Socratic philosopher and a native citizen of Akragas, a Greek city in Sicily. Empedocles' philosophy is known best for originating the Cosmogony, cosmogonic theory of the four classical elements. He also proposed forces he called Love and Strife which would mix and separate the elements, respectively. Empedocles challenged the practice of animal sacrifice and killing animals for food. He developed a distinctive doctrine of reincarnation. He is generally considered the last Greek philosopher to have recorded his ideas in verse. Some of his work survives, more than is the case for any other pre-Socratic philosopher. Empedocles' death was mythologized by ancient writers, and has been the subject of a number of literary treatments. Life The exact dates of Empedocles' birth and death are unknown, and ancient accounts of his life conflict on the exact details. However, they agree that he was born in the early 5th cent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Europe
Europe is a continent located entirely in the Northern Hemisphere and mostly in the Eastern Hemisphere. It is bordered by the Arctic Ocean to the north, the Atlantic Ocean to the west, the Mediterranean Sea to the south, and Asia to the east. Europe shares the landmass of Eurasia with Asia, and of Afro-Eurasia with both Africa and Asia. Europe is commonly considered to be Boundaries between the continents#Asia and Europe, separated from Asia by the Drainage divide, watershed of the Ural Mountains, the Ural (river), Ural River, the Caspian Sea, the Greater Caucasus, the Black Sea, and the waterway of the Bosporus, Bosporus Strait. "Europe" (pp. 68–69); "Asia" (pp. 90–91): "A commonly accepted division between Asia and Europe ... is formed by the Ural Mountains, Ural River, Caspian Sea, Caucasus Mountains, and the Black Sea with its outlets, the Bosporus and Dardanelles." Europe covers approx. , or 2% of Earth#Surface, Earth's surface (6.8% of Earth's land area), making it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Scientist
A scientist is a person who Scientific method, researches to advance knowledge in an Branches of science, area of the natural sciences. In classical antiquity, there was no real ancient analog of a modern scientist. Instead, philosophers engaged in the philosophical study of nature called natural philosophy, a precursor of natural science. Though Thales ( 624–545 BC) was arguably the first scientist for describing how cosmic events may be seen as natural, not necessarily caused by gods,Frank N. Magill''The Ancient World: Dictionary of World Biography'', Volume 1 Routledge, 2003 it was not until the 19th century in science, 19th century that the term ''scientist'' came into regular use after it was coined by the theologian, philosopher, and historian of science William Whewell in 1833. History The roles of "scientists", and their predecessors before the emergence of modern scientific disciplines, have evolved considerably over time. Scientists of different er ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Middle Ages
In the history of Europe, the Middle Ages or medieval period lasted approximately from the 5th to the late 15th centuries, similarly to the post-classical period of global history. It began with the fall of the Western Roman Empire and transitioned into the Renaissance and the Age of Discovery. The Middle Ages is the middle period of the three traditional divisions of Western history: classical antiquity, the medieval period, and the modern period. The medieval period is itself subdivided into the Early, High, and Late Middle Ages. Population decline, counterurbanisation, the collapse of centralised authority, invasions, and mass migrations of tribes, which had begun in late antiquity, continued into the Early Middle Ages. The large-scale movements of the Migration Period, including various Germanic peoples, formed new kingdoms in what remained of the Western Roman Empire. In the 7th century, North Africa and the Middle East—once part of the Byzantine Empire� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |