|
ATOX1
ATOX1 is a copper metallochaperone protein that is encoded by the ''ATOX1'' gene in humans. In mammals, ATOX1 plays a key role in copper homeostasis as it delivers copper from the cytosol to transporters ATP7A and ATP7B. Homologous proteins are found in a wide variety of eukaryotes, including ''Saccharomyces cerevisiae'' as ATX1, and all contain a conserved metal binding domain. Function ATOX1 is an abbreviation of the full name Antioxidant Protein 1. The nomenclature stems from initial characterization that showed that ATOX1 protected cells from reactive oxygen species. Since then, the primary role of ATOX1 has been established as a copper metallochaperone protein found in the cytoplasm of eukaryotes. A metallochaperone is an important protein that has metal trafficking and sequestration roles. As a metal sequestration protein, ATOX1 is capable of binding free metals ''in vivo'', in order to protect cells from generation of reactive oxygen species and mismetallation of met ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ATP7B
Wilson disease protein (WND), also known as ATP7B protein, is a copper-transporting P-type ATPase which is encoded by the ''ATP7B'' gene. The ATP7B protein is located in the trans-Golgi network of the liver and brain and balances the copper level in the body by excreting excess copper into bile and plasma. Genetic disorder of the ATP7B gene may cause Wilson's disease, a disease in which copper accumulates in tissues, leading to neurological or psychiatric issues and liver diseases. Gene Wilson disease protein is associated with ''ATP7B'' gene, approximately 80 Kb, located on human chromosome 13 and consists of 21 exons. The mRNA transcribed by ''ATP7B'' gene has a size of 7.5 Kb, and which encodes a protein of 1465 amino acids. The gene is a member of the P-type cation transport ATPase family and encodes a protein with several membrane-spanning domains, an ATPase consensus sequence, a hinge domain, a phosphorylation site, and at least two putative copper-binding sites. Thi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
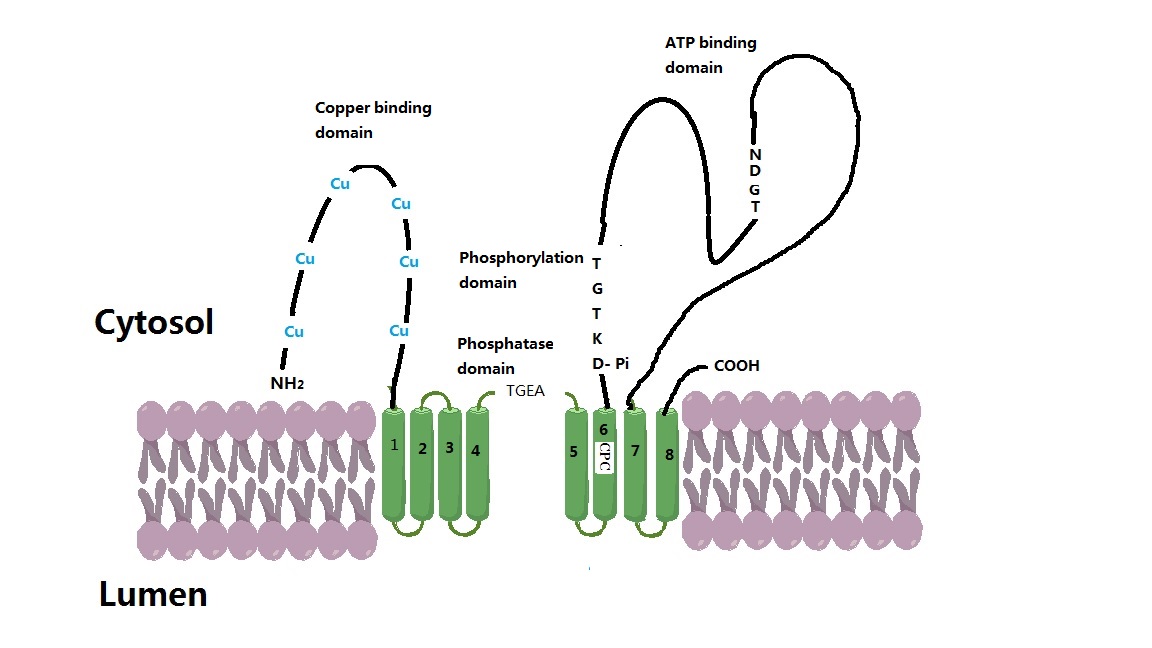
ATP7A
ATP7A, also known as Menkes' protein (MNK), is a copper-transporting P-type ATPase which uses the energy arising from ATP hydrolysis to transport Cu(I) across cell membranes. The ATP7A protein is a transmembrane protein and is expressed in the intestine and all tissues except liver. In the intestine, ATP7A regulates Cu(I) absorption in the human body by transporting Cu(I) from the small intestine into the blood. In other tissues, ATP7A shuttles between the Golgi apparatus and the cell membrane to maintain proper Cu(I) concentrations (since there is no free Cu(I) in the cell, Cu(I) ions are all tightly bound) in the cell and provides certain enzymes with Cu(I) (e.g. peptidyl-α-monooxygenase, tyrosinase, and lysyl oxidase). The X-linked, inherited, lethal genetic disorder of the ''ATP7A'' gene causes Menkes disease, a copper deficiency resulting in early childhood death. Gene The ''ATP7A'' gene is located on the long (q) arm of the X chromosome at band Xq21.1. The encoded ATP ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orange color. Copper is used as a conductor of heat and electricity, as a building material, and as a constituent of various metal alloys, such as sterling silver used in jewelry, cupronickel used to make marine hardware and coins, and constantan used in strain gauges and thermocouples for temperature measurement. Copper is one of the few metals that can occur in nature in a directly usable metallic form (native metals). This led to very early human use in several regions, from circa 8000 BC. Thousands of years later, it was the first metal to be smelted from sulfide ores, circa 5000 BC; the first metal to be cast into a shape in a mold, c. 4000 BC; and the first metal to be purposely alloyed with another metal, tin, to create br ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metalloprotein
Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins (out of ~20,000) contain zinc-binding protein domains although there may be up to 3000 human zinc metalloproteins. Abundance It is estimated that approximately half of all proteins contain a metal. In another estimate, about one quarter to one third of all proteins are proposed to require metals to carry out their functions. Thus, metalloproteins have many different functions in cells, such as storage and transport of proteins, enzymes and signal transduction proteins, or infectious diseases. The abundance of metal binding proteins may be inherent to the amino acids that proteins use, as even artificial proteins without evolutionary history will readily bind metals. Most metals in the human body are bound to proteins. For instance, the relatively high concentration of iron in the human body ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Geometry
Molecular geometry is the three-dimensional space, three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry influences several properties of a substance including its Reactivity (chemistry), reactivity, Chemical polarity, polarity, Phase (matter), phase of matter, color, magnetism and biological activity. The angles between bonds that an atom forms depend only weakly on the rest of molecule, i.e. they can be understood as approximately local and hence Transferability (chemistry), transferable properties. Determination The molecular geometry can be determined by various spectroscopy, spectroscopic methods and diffraction methods. Infrared spectroscopy, IR, Rotational spectroscopy, microwave and Raman spectroscopy can give information about the molecule geometry from the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cystine
Cystine is the oxidized derivative of the amino acid cysteine and has the formula (SCH2CH(NH2)CO2H)2. It is a white solid that is poorly soluble in water. As a residue in proteins, cystine serves two functions: a site of redox reactions and a mechanical linkage that allows proteins to retain their three-dimensional structure. Formation and reactions Structure Cystine is the disulfide derived from the amino acid cysteine. The conversion can be viewed as an oxidation: : Cystine contains a disulfide bond, two amine groups, and two carboxylic acid groups. As for other amino acids, the amine and carboxylic acid groups exist is rapid equilibrium with the ammonium-carboxylate tautomer. The great majority of the literature concerns the ''l,l-''cystine, derived from ''l''-cysteine. Other isomers include ''d,d''-cystine and the meso isomer d,l-cystine, neither of which is biologically significant. Occurrence Cystine is common in many foods such as eggs, meat, dairy products, and w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Structure
Protein structure is the molecular geometry, three-dimensional arrangement of atoms in an amino acid-chain molecule. Proteins are polymers specifically polypeptides formed from sequences of amino acids, the monomers of the polymer. A single amino acid monomer may also be called a ''residue'' indicating a repeating unit of a polymer. Proteins form by amino acids undergoing condensation reactions, in which the amino acids lose one water molecule per chemical reaction, reaction in order to attach to one another with a peptide bond. By convention, a chain under 30 amino acids is often identified as a peptide, rather than a protein. To be able to perform their biological function, proteins fold into one or more specific spatial conformations driven by a number of non-covalent interactions such as hydrogen bonding, ionic interactions, Van der Waals forces, and hydrophobic packing. To understand the functions of proteins at a molecular level, it is often necessary to determine their Pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apoenzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sequence Motif
In biology, a sequence motif is a nucleotide or amino-acid sequence pattern that is widespread and usually assumed to be related to biological function of the macromolecule. For example, an ''N''-glycosylation site motif can be defined as ''Asn, followed by anything but Pro, followed by either Ser or Thr, followed by anything but Pro residue''. Overview When a sequence motif appears in the exon of a gene, it may encode the "structural motif" of a protein; that is a stereotypical element of the overall structure of the protein. Nevertheless, motifs need not be associated with a distinctive secondary structure. " Noncoding" sequences are not translated into proteins, and nucleic acids with such motifs need not deviate from the typical shape (e.g. the "B-form" DNA double helix). Outside of gene exons, there exist regulatory sequence motifs and motifs within the " junk", such as satellite DNA. Some of these are believed to affect the shape of nucleic acids (see for exampl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferredoxin Fold
In protein structure, a ferredoxin fold is a common α+β protein fold with a signature βαββαβ secondary structure along its backbone. Structurally, the ferredoxin fold can be regarded as a long, symmetric hairpin that is wrapped once around, so that its two terminal β-strands hydrogen-bond to the central two β-strands, forming a four-stranded, antiparallel β-sheet The beta sheet, (β-sheet) (also β-pleated sheet) is a common motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone hydrogen bonds, forming a gen ... covered on one side by two α-helices. External links SCOP list of proteins with a ferredoxin-like fold References Protein folds {{protein-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transcription Factor
In molecular biology, a transcription factor (TF) (or sequence-specific DNA-binding factor) is a protein that controls the rate of transcription of genetic information from DNA to messenger RNA, by binding to a specific DNA sequence. The function of TFs is to regulate—turn on and off—genes in order to make sure that they are expressed in the desired cells at the right time and in the right amount throughout the life of the cell and the organism. Groups of TFs function in a coordinated fashion to direct cell division, cell growth, and cell death throughout life; cell migration and organization ( body plan) during embryonic development; and intermittently in response to signals from outside the cell, such as a hormone. There are up to 1600 TFs in the human genome. Transcription factors are members of the proteome as well as regulome. TFs work alone or with other proteins in a complex, by promoting (as an activator), or blocking (as a repressor) the recruitment o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |