A superalloy, or high-performance alloy, is an
alloy
An alloy is a mixture of chemical elements of which in most cases at least one is a metal, metallic element, although it is also sometimes used for mixtures of elements; herein only metallic alloys are described. Metallic alloys often have prop ...
with the ability to operate at a high fraction of its melting point.
Key characteristics of a superalloy include
mechanical strength
Mechanical may refer to:
Machine
* Machine (mechanical), a system of mechanisms that shape the actuator input to achieve a specific application of output forces and movement
* Mechanical calculator, a device used to perform the basic operations of ...
,
thermal creep deformation resistance, surface stability, and
corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engine ...
and
oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
resistance.
The crystal structure is typically
face-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties o ...
(FCC)
austenitic. Examples of such alloys are
Hastelloy,
Inconel,
Waspaloy,
Rene alloys,
Incoloy, MP98T, TMS alloys, and CMSX single crystal alloys.
Superalloy development relies on chemical and process innovations. Superalloys develop high temperature strength through
solid solution strengthening and
precipitation strengthening
Precipitation hardening, also called age hardening or particle hardening, is a heat treatment technique used to increase the Yield (engineering), yield strength of malleable materials, including most structural alloys of aluminium, magnesium, nic ...
from secondary phase precipitates such as gamma prime and
carbides
In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece.
Interstitial / Metallic carbides
The carbides of t ...
. Oxidation or corrosion resistance is provided by elements such as
aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
and
chromium
Chromium is a chemical element; it has Symbol (chemistry), symbol Cr and atomic number 24. It is the first element in Group 6 element, group 6. It is a steely-grey, Luster (mineralogy), lustrous, hard, and brittle transition metal.
Chromium ...
. Superalloys are often cast as a single crystal in order to eliminate
grain boundaries
In materials science, a grain boundary is the interface between two grains, or crystallites, in a polycrystalline material. Grain boundaries are two-dimensional crystallographic defect, defects in the crystal structure, and tend to decrease the ...
, trading in strength at low temperatures for increased resistance to thermal creep.
The primary application for such alloys is in aerospace and marine
turbine engines. Creep is typically the lifetime-limiting factor in gas turbine blades.
Superalloys have made much of very-high-temperature engineering technology possible.
Chemical development
Because these alloys are intended for high temperature applications their
creep and oxidation resistance are of primary importance.
Nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slo ...
(Ni)-based superalloys are the material of choice for these applications because of their unique γ' precipitates.
The properties of these superalloys can be tailored to a certain extent through the addition of various other elements, common or exotic, including not only
metals
A metal () is a material that, when polished or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. These properties are all associated with having electrons available at the Fermi level, as against no ...
, but also
metalloid
A metalloid is a chemical element which has a preponderance of material property, properties in between, or that are a mixture of, those of metals and Nonmetal (chemistry), nonmetals. The word metalloid comes from the Latin language, Latin ''meta ...
s and
nonmetal
In the context of the periodic table, a nonmetal is a chemical element that mostly lacks distinctive metallic properties. They range from colorless gases like hydrogen to shiny crystals like iodine. Physically, they are usually lighter (less ...
s;
chromium
Chromium is a chemical element; it has Symbol (chemistry), symbol Cr and atomic number 24. It is the first element in Group 6 element, group 6. It is a steely-grey, Luster (mineralogy), lustrous, hard, and brittle transition metal.
Chromium ...
,
iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
,
cobalt
Cobalt is a chemical element; it has Symbol (chemistry), symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. ...
,
molybdenum
Molybdenum is a chemical element; it has Symbol (chemistry), symbol Mo (from Neo-Latin ''molybdaenum'') and atomic number 42. The name derived from Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals hav ...
,
tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
,
tantalum
Tantalum is a chemical element; it has Symbol (chemistry), symbol Ta and atomic number 73. It is named after Tantalus, a figure in Greek mythology. Tantalum is a very hard, ductility, ductile, lustre (mineralogy), lustrous, blue-gray transition ...
,
aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
,
titanium
Titanium is a chemical element; it has symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in ...
,
zirconium
Zirconium is a chemical element; it has Symbol (chemistry), symbol Zr and atomic number 40. First identified in 1789, isolated in impure form in 1824, and manufactured at scale by 1925, pure zirconium is a lustrous transition metal with a greyis ...
,
niobium
Niobium is a chemical element; it has chemical symbol, symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and Ductility, ductile transition metal. Pure niobium has a Mohs scale of mineral hardness, Mohs h ...
,
rhenium
Rhenium is a chemical element; it has symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one ...
,
yttrium
Yttrium is a chemical element; it has Symbol (chemistry), symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a "rare-earth element". Yttrium is almost a ...
,
vanadium
Vanadium is a chemical element; it has Symbol (chemistry), symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an ...
,
carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
,
boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
or
hafnium
Hafnium is a chemical element; it has symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dm ...
are some examples of the alloying additions used. Each addition serves a particular purpose in optimizing properties.
Creep resistance is dependent, in part, on slowing the speed of
dislocation
In materials science, a dislocation or Taylor's dislocation is a linear crystallographic defect or irregularity within a crystal structure that contains an abrupt change in the arrangement of atoms. The movement of dislocations allow atoms to sli ...
motion within a crystal structure. In modern Ni-based superalloys, the γ'-Ni
3(Al,Ti)
phase acts as a barrier to dislocation. For this reason, this γ;'
intermetallic
An intermetallic (also called intermetallic compound, intermetallic alloy, ordered intermetallic alloy, long-range-ordered alloy) is a type of metallic alloy that forms an ordered solid-state compound between two or more metallic elements. Inte ...
phase, when present in high volume fractions, increases the strength of these alloys due to its ordered nature and high coherency with the γ matrix. The chemical additions of
aluminum
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
and
titanium
Titanium is a chemical element; it has symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in ...
promote the creation of the γ' phase. The γ' phase size can be precisely controlled by careful precipitation strengthening heat treatments. Many superalloys are produced using a two-phase heat treatment that creates a dispersion of cuboidal γ' particles known as the primary phase, with a fine dispersion between these known as secondary γ'. In order to improve the oxidation resistance of these alloys,
Al,
Cr,
B, and
Y are added. The
Al and
Cr form oxide layers that passivate the surface and protect the superalloy from further oxidation while
B and
Y are used to improve the adhesion of this oxide scale to the substrate.
Cr,
Fe,
Co,
Mo and
Re all preferentially partition to the γ matrix while
Al,
Ti,
Nb,
Ta, and
V preferentially partition to the γ' precipitates and solid solution strengthen the matrix and precipitates respectively. In addition to solid solution strengthening, if grain boundaries are present, certain elements are chosen for grain boundary strengthening.
B and
Zr tend to segregate to the grain boundaries which reduces the grain boundary energy and results in better grain boundary cohesion and ductility. Another form of grain boundary strengthening is achieved through the addition of
C and a carbide former, such as
Cr,
Mo,
W,
Nb,
Ta,
Ti, or
Hf, which drives precipitation of carbides at grain boundaries and thereby reduces grain boundary sliding.
Phase formation
Adding elements is usually helpful because of solid solution strengthening, but can result in unwanted precipitation. Precipitates can be classified as geometrically close-packed (GCP),
topologically close-packed (TCP), or carbides. GCP phases usually benefit mechanical properties, but TCP phases are often deleterious. Because TCP phases are not truly close packed, they have few slip systems and are brittle. Also they "scavenge" elements from GCP phases. Many elements that are good for forming γ' or have great solid solution strengthening may precipitate TCPs. The proper balance promotes GCPs while avoiding TCPs.
TCP phase formation areas are weak because they:
* have inherently poor mechanical properties
* are incoherent with the γ matrix
* are surrounded by a "depletion zone" where there is no γ'
* usually form sharp plate or needle-like morphologies which nucleate cracks
The main GCP phase is γ'. Almost all superalloys are Ni-based because of this phase. γ' is an ordered L1 (pronounced L-one-two), which means it has a certain atom on the face of the unit cell, and a certain atom on the corners of the unit cell. Ni-based superalloys usually present Ni on the faces and Ti or Al on the corners.
Another "good" GCP phase is γ
''. It is also coherent with γ, but it dissolves at high temperatures.
Families of superalloys
Ni-based
History
The United States became interested in gas turbine development around 1905.
From 1910-1915, austenitic ( γ phase) stainless steels were developed to survive high temperatures in gas turbines. By 1929, 80Ni-20Cr alloy was the norm, with small additions of Ti and Al. Although early metallurgists did not know it yet, they were forming small γ' precipitates in Ni-based superalloys. These alloys quickly surpassed Fe- and Co-based superalloys, which were strengthened by carbides and solid solution strengthening.
Although Cr was great for protecting the alloys from oxidation and corrosion up to 700 °C, metallurgists began decreasing Cr in favor of Al, which had oxidation resistance at much higher temperatures. The lack of Cr caused issues with hot corrosion, so coatings needed to be developed.
Around 1950,
vacuum melting became commercialized, which allowed metallurgists to create higher purity alloys with more precise composition.
In the 60s and 70s, metallurgists changed focus from alloy chemistry to alloy processing.
Directional solidification was developed to allow columnar or even single-crystal turbine blades.
Oxide dispersion strengthening could obtain very fine grains and
superplasticity.
Phases
* Gamma (γ): This phase composes the matrix of Ni-based superalloy. It is a solid solution fcc austenitic phase of the alloying elements.
The alloying elements most found in commercial Ni-based alloys are, C, Cr, Mo, W, Nb, Fe, Ti, Al, V, and Ta. During the formation of these materials, as they cool from the melt, carbides precipitate, and at even lower temperatures γ' phase precipitates.
* Gamma prime (γ'): This phase constitutes the precipitate used to strengthen the alloy. It is an
intermetallic
An intermetallic (also called intermetallic compound, intermetallic alloy, ordered intermetallic alloy, long-range-ordered alloy) is a type of metallic alloy that forms an ordered solid-state compound between two or more metallic elements. Inte ...
phase based on Ni
3(Ti,Al) which have an ordered FCC L1
2 structure.
The γ' phase is coherent with the matrix of the superalloy having a lattice parameter that varies by around 0.5%. Ni
3(Ti,Al) are ordered systems with Ni atoms at the cube faces and either Al or Ti atoms at the cube edges. As particles of γ' precipitates aggregate, they decrease their energy states by aligning along the <100> directions forming cuboidal structures.
This phase has a window of instability between 600 °C and 850 °C, inside of which γ' will transform into the HCP η phase. For applications at temperatures below 650 °C, the γ" phase can be utilized for strengthening.
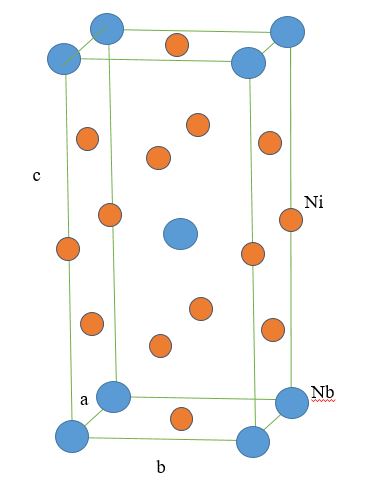
* Gamma double prime (γ"): This phase typically is Ni
3Nb or Ni
3V and is used to strengthen Ni-based superalloys at lower temperatures (<650 °C) relative to γ'. The crystal structure of γ" is
body-centered tetragonal (BCT), and the phase precipitates as 60 nm by 10 nm discs with the (001) planes in γ" parallel to the family in γ. These
anisotropic
Anisotropy () is the structural property of non-uniformity in different directions, as opposed to isotropy. An anisotropic object or pattern has properties that differ according to direction of measurement. For example, many materials exhibit ver ...
discs form as a result of
lattice mismatch between the
BCT precipitate and the
FCC matrix. This
lattice mismatch leads to high
coherency strains which, together with order hardening, are the primary strengthening mechanisms. The γ" phase is unstable above approximately 650 °C.
[Dunand, David C. "Materials Science & Engineering 435: High Temperature Materials". Northwestern University, Evanston. 25 February 2016. Lecture.]
* Carbide phases: Carbide formation is usually deleterious although in Ni-based superalloys they are used to stabilize the structure of the material against deformation at high temperatures. Carbides form at the grain boundaries, inhibiting grain boundary motion.
*Topologically close-packed (TCP) phases: The term
"TCP phase" refers to any member of a family of phases (including the σ phase, the χ phase, the μ phase, and the
Laves phase
Laves phases are intermetallic phase (matter), phases that have composition AB2 and are named for Fritz Laves who first described them. The phases are classified on the basis of geometry alone. While the problem of Close-packing of equal spheres ...
), which are not atomically close-packed but possess some close-packed planes with
HCP stacking. TCP phases tend to be highly brittle and deplete the γ matrix of strengthening,
solid solution
A solid solution, a term popularly used for metals, is a homogeneous mixture of two compounds in solid state and having a single crystal structure. Many examples can be found in metallurgy, geology, and solid-state chemistry. The word "solutio ...
refractory elements (including Cr, Co, W, and Mo). These phases form as a result of kinetics after long periods of time (thousands of hours) at high temperatures (>750 °C).
Co-based
Co-based superalloys depend on carbide precipitation and solid solution strengthening for mechanical properties. While these strengthening mechanisms are inferior to gamma prime (γ') precipitation strengthening,
cobalt has a higher melting point than nickel and has superior hot corrosion resistance and thermal fatigue. As a result, carbide-strengthened Co-based superalloys are used in lower stress, higher temperature applications such as stationary vanes in gas turbines.
Co's γ/γ' microstructure was rediscovered and published in 2006 by Sato et al.
That γ' phase was Co
3(Al, W). Mo, Ti, Nb, V, and Ta partition to the γ' phase, while Fe, Mn, and Cr partition to the matrix γ.
The next family of Co-based superalloys was discovered in 2015 by Makineni et al. This family has a similar γ/γ' microstructure, but is W-free and has a γ' phase of Co
3(Al,Mo,Nb).
Since W is heavy, its elimination makes Co-based alloys increasingly viable in turbines for aircraft, where low density is especially valued.
The most recently discovered family of superalloys was computationally predicted by Nyshadham et al. in 2017, and demonstrated by Reyes Tirado et al. in 2018.
This γ' phase is W free and has the composition Co
3(Nb,V) and Co
3(Ta,V).
Phases
*Gamma (γ): This is the matrix phase. While Co-based superalloys are less-used commercially, alloying elements include C, Cr, W, Ni, Ti, Al, Ir, and Ta.
As in stainless steels, Chromium is used (occasionally up to 20 wt.%) to improve resistance to oxidation and corrosion via the formation of a Cr
2O
3 passive layer, which is critical for use in gas turbines, but also provides solid-solution strengthening due to the mismatch in the atomic radii of Co and Cr, and precipitation hardening due to the formation of MC-type carbides.
* Gamma Prime (γ'): Constitutes the precipitate used to strengthen the alloy. It is usually close-packed with a L1
2 structure of Co
3Ti or FCC Co
3Ta, though both W and Al integrate into these cuboidal precipitates. Ta, Nb, and Ti integrate into the γ' phase and are stabilize it at high temperatures.
* Carbide Phases: Carbides strengthen the alloy through precipitation hardening but decrease low-temperature ductility.
* Topologically Close-Packed (TCP) phases may appear in some Co-based superalloys, but embrittle the alloy and are thus undesirable.
Fe-based
Steel superalloys are of interest because some present creep and oxidation resistance similar to Ni-based superalloys, at far less cost.
Gamma (γ): Fe-based alloys feature a matrix phase of austenite iron (FCC). Alloying elements include: Al, B, C, Co, Cr, Mo, Ni, Nb, Si, Ti, W, and Y. Al (oxidation benefits) must be kept at low weight fractions (wt.%) because Al stabilizes a ferritic (BCC) primary phase matrix, which is undesirable, as it is inferior to the high temperature strength exhibited by an austenitic (FCC) primary phase matrix.
Gamma-prime (γ'): This phase is introduced as precipitates to strengthen the alloy. γ'-Ni3Al precipitates can be introduced with the proper balance of Al, Ni, Nb, and Ti additions.
Microstructure
The two major types of austenitic stainless steels are characterized by the oxide layer that forms on the steel surface: either chromia-forming or alumina-forming. Cr-forming stainless steel is the most common type. However, Cr-forming steels do not exhibit high creep resistance at high temperatures, especially in environments with water vapor. Exposure to water vapor at high temperatures can increase internal oxidation in Cr-forming alloys and rapid formation of volatile Cr (oxy)hydroxides, both of which can reduce durability and lifetime.
Al-forming austenitic stainless steels feature a single-phase matrix of austenite iron (FCC) with an Al-oxide at the surface of the steel. Al is more thermodynamically stable in oxygen than Cr. More commonly, however, precipitate phases are introduced to increase strength and creep resistance. In Al-forming steels, NiAl precipitates are introduced to act as Al reservoirs to maintain the protective alumina layer. In addition, Nb and Cr additions help form and stabilize Al by increasing precipitate volume fractions of NiAl.
At least 5 grades of alumina-forming austenitic (AFA) alloys, with different operating temperatures at oxidation in air + 10% water vapor have been realized:
* AFA Grade: (50-60)Fe-(20-25)Ni-(14-15)Cr-(2.5-3.5)Al-(1-3)Nb wt.% base
** 750-800 °C operating temperatures at oxidation in air + 10% water vapor
* Low Nickel AFA Grade: 63Fe-12Ni-14Cr-2.5Al-0.6Nb-5Mn3Cu wt.% base
** 650 °C operating temperatures at oxidation in air + 10% water vapor
* High Performance AFA Grade: (45-55)Fe-(25-30)Ni-(14-15)Cr(3.5-4.5)Al-(1-3)Nb-(0.02-0.1)Hf/Y wt.% base
** 850-900 °C operating temperatures at oxidation in air + 10% water vapor
* Cast AFA Grade: (35-50)Fe-(25-35)Ni-14Cr-(3.5-4)Al-1Nb wt.% base
** 750-1100 °C operating temperatures at oxidation in air + 10% water vapor, depending upon Ni wt.%
* AFA superalloy (40-50)Fe-(30-35)Ni-(14-19)Cr-(2.5-3.5)Al-3Nb
** 750-850 °C operating temperatures at oxidation in air + 10% water vapor
Operating temperatures with oxidation in air and no water vapor are expected to be higher. In addition, an AFA superalloy grade exhibits creep strength approaching that of nickel alloy UNS N06617.
Microstructure
In pure Ni
3Al phase Al
atoms
Atoms are the basic particles of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other ...
are placed at the vertices of the cubic cell and form sublattice A. Ni atoms are located at centers of the faces and form sublattice B. The phase is not strictly
stoichiometric
Stoichiometry () is the relationships between the masses of reactants and products before, during, and following chemical reactions.
Stoichiometry is based on the law of conservation of mass; the total mass of reactants must equal the total m ...
. An excess of vacancies in one of the sublattices may exist, which leads to deviations from stoichiometry. Sublattices A and B of the γ' phase can solute a considerable proportion of other elements. The alloying elements are dissolved in the γ phase. The γ' phase hardens the alloy through the
yield strength anomaly.
Dislocation
In materials science, a dislocation or Taylor's dislocation is a linear crystallographic defect or irregularity within a crystal structure that contains an abrupt change in the arrangement of atoms. The movement of dislocations allow atoms to sli ...
s dissociate in the γ' phase, leading to the formation of an
anti-phase boundary.

To give an example, consider a dislocation with a
burgers vector
In materials science, the Burgers vector, named after Dutch physicist Jan Burgers, is a Vector (geometric), vector, often denoted as , that represents the Magnitude (vector), magnitude and direction of the lattice distortion resulting from a dislo ...
of
 * Gamma double prime (γ"): This phase typically is Ni3Nb or Ni3V and is used to strengthen Ni-based superalloys at lower temperatures (<650 °C) relative to γ'. The crystal structure of γ" is body-centered tetragonal (BCT), and the phase precipitates as 60 nm by 10 nm discs with the (001) planes in γ" parallel to the family in γ. These
* Gamma double prime (γ"): This phase typically is Ni3Nb or Ni3V and is used to strengthen Ni-based superalloys at lower temperatures (<650 °C) relative to γ'. The crystal structure of γ" is body-centered tetragonal (BCT), and the phase precipitates as 60 nm by 10 nm discs with the (001) planes in γ" parallel to the family in γ. These  To give an example, consider a dislocation with a
To give an example, consider a dislocation with a  To give an example, consider a dislocation with a
To give an example, consider a dislocation with a