|
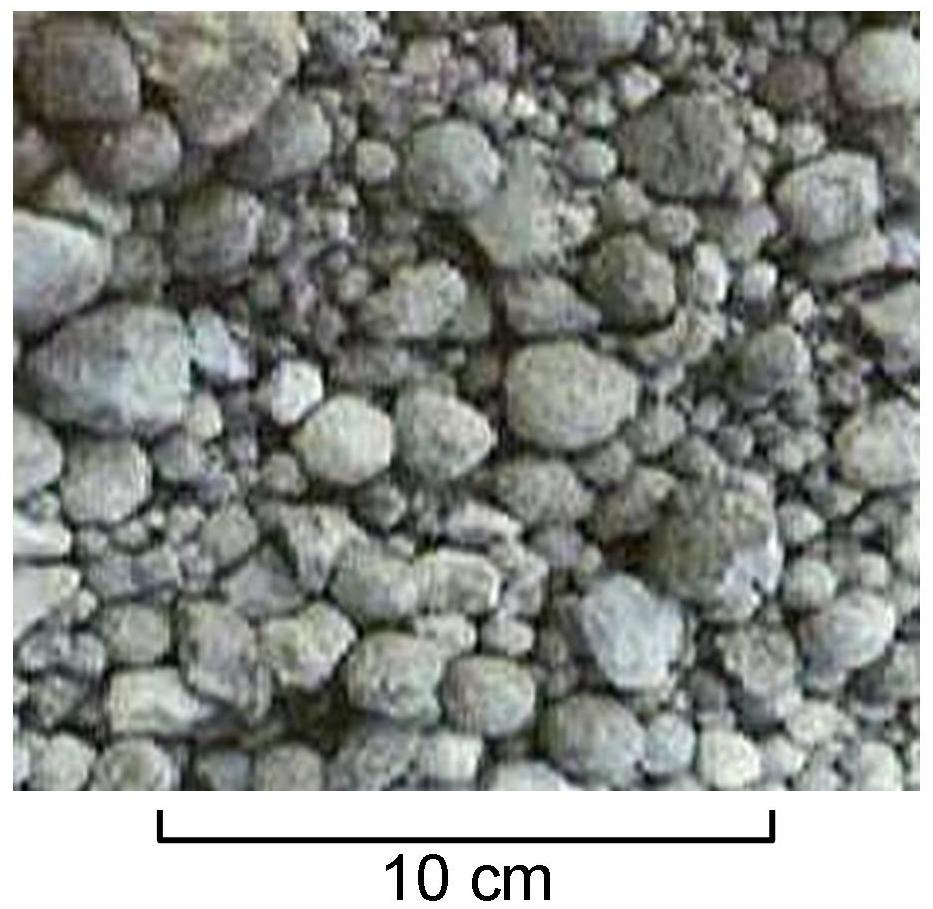
Sintering
Sintering or frittage is the process of compacting and forming a solid mass of material by pressure or heat without melting it to the point of liquefaction. Sintering happens as part of a manufacturing process used with metals, ceramics, plastics, and other materials. The atoms/molecules in the sintered material diffuse across the boundaries of the particles, fusing the particles together and creating a solid piece. Since the sintering temperature does not have to reach the melting point of the material, sintering is often chosen as the shaping process for materials with extremely high melting points, such as tungsten and molybdenum. The study of sintering in metallurgy, metallurgical powder-related processes is known as powder metallurgy. An example of sintering can be observed when ice cubes in a glass of water adhere to each other, which is driven by the temperature difference between the water and the ice. Examples of pressure-driven sintering are the compacting of snowfa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sintering Diagram Vector
Sintering or frittage is the process of compacting and forming a solid mass of material by pressure or heat without melting it to the point of liquefaction. Sintering happens as part of a manufacturing process used with metals, ceramics, plastics, and other materials. The atoms/molecules in the sintered material diffuse across the boundaries of the particles, fusing the particles together and creating a solid piece. Since the sintering temperature does not have to reach the melting point of the material, sintering is often chosen as the shaping process for materials with extremely high melting points, such as tungsten and molybdenum. The study of sintering in metallurgical powder-related processes is known as powder metallurgy. An example of sintering can be observed when ice cubes in a glass of water adhere to each other, which is driven by the temperature difference between the water and the ice. Examples of pressure-driven sintering are the compacting of snowfall to a gl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Powder Metallurgy
Powder metallurgy (PM) is a term covering a wide range of ways in which materials or components are made from metal powders. PM processes are sometimes used to reduce or eliminate the need for subtractive manufacturing, subtractive processes in manufacturing, lowering material losses and reducing the cost of the final product. This occurs especially often with small metal parts, like gears for small machines. Some porous products, allowing liquid or gas to permeate them, are produced in this way. They are also used when melting a material is impractical, due to it having a high melting point, or an alloy of two mutually insoluble materials, such as a mixture of copper and graphite. In this way, powder metallurgy can be used to make unique materials impossible to get from melting or forming in other ways. A very important product of this type is tungsten carbide. Tungsten carbide is used to cut and form other metals and is made from tungsten carbide particles bonded with cobalt. Tu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ceramic
A ceramic is any of the various hard, brittle, heat-resistant, and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcelain, and brick. The earliest ceramics made by humans were fired clay bricks used for building house walls and other structures. Other pottery objects such as pots, vessels, vases and figurines were made from clay, either by itself or mixed with other materials like silica, hardened by sintering in fire. Later, ceramics were glazed and fired to create smooth, colored surfaces, decreasing porosity through the use of glassy, amorphous ceramic coatings on top of the crystalline ceramic substrates. Ceramics now include domestic, industrial, and building products, as well as a wide range of materials developed for use in advanced ceramic engineering, such as semiconductors. The word '' ceramic'' comes from the Ancient Greek word (), meaning ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first isolated as a metal in 1783. Its important ores include scheelite and wolframite, the latter lending the element its alternative name. The free element is remarkable for its robustness, especially the fact that it has the highest melting point of all known elements, melting at . It also has the highest boiling point, at . Its density is 19.254 g/cm3, comparable with that of uranium and gold, and much higher (about 1.7 times) than that of lead. Polycrystalline tungsten is an intrinsically brittle and hard material (under standard conditions, when uncombined), making it difficult to work into metal. However, pure single-crystalline tungsten is more ductile and can be cut with a hard-steel hacksaw. Tungsten occurs in many alloys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alumina
Aluminium oxide (or aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula . It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium oxide. It is commonly called alumina and may also be called aloxide, aloxite, ALOX or alundum in various forms and applications and alumina is refined from bauxite. It occurs naturally in its crystalline polymorphic phase α-Al2O3 as the mineral corundum, varieties of which form the precious gemstones ruby and sapphire,which have an alumina content approaching 100%. Al2O3 is used as feedstock to produce aluminium metal, as an abrasive owing to its hardness, and as a refractory material owing to its high melting point. Natural occurrence Corundum is the most common naturally occurring crystalline form of aluminium oxide. Rubies and sapphires are gem-quality forms of corundum, which owe their characteristic colours to trace impurities. Rubies are given their ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pottery
Pottery is the process and the products of forming vessels and other objects with clay and other raw materials, which are fired at high temperatures to give them a hard and durable form. The place where such wares are made by a ''potter'' is also called a ''pottery'' (plural ''potteries''). The definition of ''pottery'', used by the ASTM International, is "all fired ceramic wares that contain clay when formed, except technical, structural, and refractory products". End applications include tableware, ceramic art, decorative ware, toilet, sanitary ware, and in technology and industry such as Insulator (electricity), electrical insulators and laboratory ware. In art history and archaeology, especially of ancient and prehistoric periods, pottery often means only vessels, and sculpture, sculpted figurines of the same material are called terracottas. Pottery is one of the Timeline of historic inventions, oldest human inventions, originating before the Neolithic, Neolithic period, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melting Point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilibrium, equilibrium. The melting point of a substance depends on pressure and is usually specified at a Standard temperature and pressure, standard pressure such as 1 Atmosphere (unit), atmosphere or 100 Pascal (unit), kPa. When considered as the temperature of the reverse change from liquid to solid, it is referred to as the freezing point or crystallization point. Because of the ability of substances to Supercooling, supercool, the freezing point can easily appear to be below its actual value. When the "characteristic freezing point" of a substance is determined, in fact, the actual methodology is almost always "the principle of observing the disappearance rather than the formation of ice, that is, the #Melting point measurements, melting ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molybdenum
Molybdenum is a chemical element; it has Symbol (chemistry), symbol Mo (from Neo-Latin ''molybdaenum'') and atomic number 42. The name derived from Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals have been known throughout history, but the element was discovered (in the sense of differentiating it as a new entity from the mineral salts of other metals) in 1778 by Carl Wilhelm Scheele. The metal was first isolated in 1781 by Peter Jacob Hjelm. Molybdenum does not occur naturally as a Native metal, free metal on Earth; in its minerals, it is found only in oxidation state, oxidized states. The free element, a silvery metal with a grey cast, has the List of elements by melting point, sixth-highest melting point of any element. It readily forms hard, stable carbides in alloys, and for this reason most of the world production of the element (about 80%) is used in steel alloys, including high-strength alloys and superalloys. Most molybdenum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid
Solid is a state of matter where molecules are closely packed and can not slide past each other. Solids resist compression, expansion, or external forces that would alter its shape, with the degree to which they are resisted dependent upon the specific material under consideration. Solids also always possess the least amount of kinetic energy per atom/molecule relative to other phases or, equivalently stated, solids are formed when matter in the liquid / gas phase is cooled below a certain temperature. This temperature is called the melting point of that substance and is an intrinsic property, i.e. independent of how much of the matter there is. All matter in solids can be arranged on a microscopic scale under certain conditions. Solids are characterized by structural rigidity and resistance to applied external forces and pressure. Unlike liquids, solids do not flow to take on the shape of their container, nor do they expand to fill the entire available volume like a gas. Much ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass
Glass is an amorphous (non-crystalline solid, non-crystalline) solid. Because it is often transparency and translucency, transparent and chemically inert, glass has found widespread practical, technological, and decorative use in window panes, tableware, and optics. Some common objects made of glass are named after the material, e.g., a Tumbler (glass), "glass" for drinking, "glasses" for vision correction, and a "magnifying glass". Glass is most often formed by rapid cooling (quenching) of the Melting, molten form. Some glasses such as volcanic glass are naturally occurring, and obsidian has been used to make arrowheads and knives since the Stone Age. Archaeological evidence suggests glassmaking dates back to at least 3600 BC in Mesopotamia, Ancient Egypt, Egypt, or Syria. The earliest known glass objects were beads, perhaps created accidentally during metalworking or the production of faience, which is a form of pottery using lead glazes. Due to its ease of formability int ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vitrification
Vitrification (, via French ') is the full or partial transformation of a substance into a glass, that is to say, a non- crystalline or amorphous solid. Glasses differ from liquids structurally and glasses possess a higher degree of connectivity with the same Hausdorff dimensionality of bonds as crystals: dimH = 3. In the production of ceramics, vitrification is responsible for their impermeability to water. Vitrification is usually achieved by heating materials until they liquify, then cooling the liquid, often rapidly, so that it passes through the glass transition to form a glassy solid. Certain chemical reactions also result in glasses. In terms of chemistry, vitrification is characteristic for amorphous materials or disordered systems and occurs when bonding between elementary particles (atoms, molecules, forming blocks) becomes higher than a certain threshold value. Thermal fluctuations break the bonds; therefore, the lower the temperature, the higher the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |