Polyurethane Synthesis on:
[Wikipedia]
[Google]
[Amazon]

 Polyurethane (; often abbreviated PUR and PU) refers to a class of polymers composed of
Polyurethane (; often abbreviated PUR and PU) refers to a class of polymers composed of
 Otto Bayer and his coworkers at
Otto Bayer and his coworkers at
 Aliphatic and cycloaliphatic isocyanates are used in smaller quantities, most often in coatings and other applications where color and transparency are important since polyurethanes made with aromatic isocyanates tend to darken on exposure to light. The most important aliphatic and cycloaliphatic isocyanates are 1,6-hexamethylene diisocyanate (HDI), 1-isocyanato-3-isocyanatomethyl-3,5,5-trimethyl-cyclohexane ( isophorone diisocyanate, IPDI), and 4,4′-diisocyanato dicyclohexylmethane (H12MDI or hydrogenated MDI).
Aliphatic and cycloaliphatic isocyanates are used in smaller quantities, most often in coatings and other applications where color and transparency are important since polyurethanes made with aromatic isocyanates tend to darken on exposure to light. The most important aliphatic and cycloaliphatic isocyanates are 1,6-hexamethylene diisocyanate (HDI), 1-isocyanato-3-isocyanatomethyl-3,5,5-trimethyl-cyclohexane ( isophorone diisocyanate, IPDI), and 4,4′-diisocyanato dicyclohexylmethane (H12MDI or hydrogenated MDI).
 Polyurethane (; often abbreviated PUR and PU) refers to a class of polymers composed of
Polyurethane (; often abbreviated PUR and PU) refers to a class of polymers composed of organic
Organic may refer to:
* Organic, of or relating to an organism, a living entity
* Organic, of or relating to an anatomical organ
Chemistry
* Organic matter, matter that has come from a once-living organism, is capable of decay or is the product ...
units joined by carbamate
In organic chemistry, a carbamate is a category of organic compounds with the general formula and structure , which are formally derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally o ...
(urethane) links. In contrast to other common polymers such as polyethylene and polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It is a ...
, polyurethane is produced from a wide range of starting materials. This chemical variety produces polyurethanes with different chemical structures leading to many different applications. These include rigid and flexible foams, varnishes and coatings, adhesives, electrical potting compounds, and fibers such as spandex and PUL. Foams are the largest application accounting for 67% of all polyurethane produced in 2016.
A polyurethane is typically produced by reacting an isocyanate with a polyol. Since a polyurethane contains two types of monomers, which polymerize one after the other, they are classed as alternating copolymers. Both the isocyanates and polyols used to make a polyurethane contain two or more functional groups per molecule.
Global production in 2019 was 25 million metric tonnes, accounting for about 6% of all polymers produced in that year. Polyurethane is a commodity plastic.
History
 Otto Bayer and his coworkers at
Otto Bayer and his coworkers at IG Farben
Interessengemeinschaft Farbenindustrie AG (), commonly known as IG Farben (German for 'IG Dyestuffs'), was a German chemical and pharmaceutical conglomerate (company), conglomerate. Formed in 1925 from a merger of six chemical companies—BASF, ...
in Leverkusen, Germany, first made polyurethanes in 1937. The new polymers had some advantages over existing plastics that were made by polymerizing olefins or by polycondensation, and were not covered by patents obtained by Wallace Carothers on polyester
Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include natural ...
s. Early work focused on the production of fibers and flexible foams and PUs were applied on a limited scale as aircraft coating during World War II. Polyisocyanates became commercially available in 1952, and production of flexible polyurethane foam began in 1954 by combining toluene diisocyanate (TDI) and polyester polyols. These materials were also used to produce rigid foams, gum rubber, and elastomer
An elastomer is a polymer with viscoelasticity (i.e. both viscosity and elasticity) and with weak intermolecular forces, generally low Young's modulus and high failure strain compared with other materials. The term, a portmanteau of ''elastic p ...
s. Linear fibers were produced from hexamethylene diisocyanate (HDI) and 1,4-Butanediol
1,4-Butanediol, colloquially known as BD or BDO, is a primary alcohol, and an organic compound, with the formula HOCH2CH2CH2CH2OH. It is a colorless viscous liquid. It is one of four stable isomers of butanediol.
Synthesis
In industrial sy ...
(BDO).
DuPont
DuPont de Nemours, Inc., commonly shortened to DuPont, is an American multinational chemical company first formed in 1802 by French-American chemist and industrialist Éleuthère Irénée du Pont de Nemours. The company played a major role in ...
introduced polyethers, specifically poly(tetramethylene ether) glycol, in 1956. BASF and Dow Chemical
The Dow Chemical Company, officially Dow Inc., is an American multinational chemical corporation headquartered in Midland, Michigan, United States. The company is among the three largest chemical producers in the world.
Dow manufactures plastics ...
introduced polyalkylene glycols in 1957. Polyether polyols were cheaper, easier to handle and more water-resistant than polyester polyols. Union Carbide and Mobay
Mobay Chemical Corporation, based in Pittsburgh, Pennsylvania, was a joint venture of Monsanto Company and Bayer to market polyurethanes in the United States. Founded in 1954, Bayer bought out Monsanto's shares in the company in the 1970s.
Moba ...
, a U.S. Monsanto/Bayer
Bayer AG (, commonly pronounced ; ) is a German multinational corporation, multinational pharmaceutical and biotechnology company and one of the largest pharmaceutical companies in the world. Headquartered in Leverkusen, Bayer's areas of busi ...
joint venture, also began making polyurethane chemicals. In 1960 more than 45,000 metric tons of flexible polyurethane foams were produced. The availability of chlorofluoroalkane
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F), produced as volatile derivatives of methane, ethane, and propan ...
blowing agents, inexpensive polyether polyols, and methylene diphenyl diisocyanate (MDI) allowed polyurethane rigid foams to be used as high-performance insulation materials. In 1967, urethane-modified polyisocyanurate
Polyisocyanurate (), also referred to as PIR, polyiso, or ISO, is a thermoset plastic typically produced as a foam and used as rigid thermal insulation. The starting materials are similar to those used in polyurethane (PUR) except that the prop ...
rigid foams were introduced, offering even better thermal stability and flammability resistance. During the 1960s, automotive interior safety components, such as instrument and door panels, were produced by back-filling thermoplastic skins with semi-rigid foam.
In 1969, Bayer exhibited an all-plastic car in Düsseldorf, Germany. Parts of this car, such as the fascia
A fascia (; plural fasciae or fascias; adjective fascial; from Latin: "band") is a band or sheet of connective tissue, primarily collagen, beneath the skin that attaches to, stabilizes, encloses, and separates muscles and other internal organs. ...
and body panels, were manufactured using a new process called reaction injection molding (RIM), in which the reactants were mixed and then injected into a mold. The addition of fillers, such as milled glass, mica
Micas ( ) are a group of silicate minerals whose outstanding physical characteristic is that individual mica crystals can easily be split into extremely thin elastic plates. This characteristic is described as perfect basal cleavage. Mica is ...
, and processed mineral fibers, gave rise to reinforced RIM (RRIM), which provided improvements in flexural modulus (stiffness), reduction in coefficient of thermal expansion and better thermal stability. This technology was used to make the first plastic-body automobile in the United States, the Pontiac Fiero, in 1983. Further increases in stiffness were obtained by incorporating pre-placed glass mats into the RIM mold cavity, also known broadly as resin injection molding, or structural RIM.
Starting in the early 1980s, water-blown microcellular flexible foams were used to mold gaskets for automotive panels and air-filter seals, replacing PVC polymers. Polyurethane foams are used in many automotive applications including seating, head and arm rests, and headliners.
Polyurethane foam (including foam rubber) is sometimes made using small amounts of blowing agents to give less dense foam, better cushioning/energy absorption or thermal insulation. In the early 1990s, because of their impact on ozone depletion
Ozone depletion consists of two related events observed since the late 1970s: a steady lowering of about four percent in the total amount of ozone in Earth's atmosphere, and a much larger springtime decrease in stratospheric ozone (the ozone l ...
, the Montreal Protocol restricted the use of many chlorine-containing blowing agents, such as trichlorofluoromethane (CFC-11). By the late 1990s, blowing agents such as carbon dioxide, pentane
Pentane is an organic compound with the formula C5H12—that is, an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, pentane means exclusively the ' ...
, 1,1,1,2-tetrafluoroethane
1,1,1,2-Tetrafluoroethane (also known as norflurane (INN), R-134a, Freon 134a, Forane 134a, Genetron 134a, Green Gas, Florasol 134a, Suva 134a, or HFC-134a) is a hydrofluorocarbon (HFC) and haloalkane refrigerant with thermodynamic properties s ...
(HFC-134a) and 1,1,1,3,3-pentafluoropropane (HFC-245fa) were widely used in North America and the EU, although chlorinated blowing agents remained in use in many developing countries. Later, HFC-134a was also banned due to high ODP and GWP readings, and HFC - 141B was introduced in early 2000s as an alternate blowing agent in developing nations.
Chemistry
Polyurethanes are produced by reacting di isocyanates with polyols,''n'' ≥ 2 in the presence of a catalyst, or upon exposure to ultraviolet light. Common catalysts include tertiary amines, such as DABCO, or metallic soaps, such as dibutyltin dilaurate. Thestoichiometry
Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions.
Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equal ...
of the starting materials must be carefully controlled as excess isocyanate can trimerise, leading to the formation of rigid polyisocyanurate
Polyisocyanurate (), also referred to as PIR, polyiso, or ISO, is a thermoset plastic typically produced as a foam and used as rigid thermal insulation. The starting materials are similar to those used in polyurethane (PUR) except that the prop ...
s. The polymer usually has a highly crosslinked molecular structure, resulting in a thermosetting material which does not melt on heating; although some thermoplastic polyurethanes are also produced.
The most common application of polyurethane is as solid foams, which requires the presence of a gas, or blowing agent, during the polymerization step. This is commonly achieved by adding small amounts of water, which reacts with isocyanates to form CO2 gas and an amine, via an unstable carbamic acid group. The amine produced can also react with isocyanates to form urea groups, and as such the polymer will contain both these and urethane linkers. The urea is not very soluble in the reaction mixture and tends to form separate "hard segment" phases consisting mostly of polyurea. The concentration and organization of these polyurea phases can have a significant impact on the properties of the foam.
The type of foam produced can be controlled by regulating the amount of blowing agent and also by the addition of various surfactant
Surfactants are chemical compounds that decrease the surface tension between two liquids, between a gas and a liquid, or interfacial tension between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming ...
s which change the rheology of the polymerising mixture. Foams can be either "closed-cell", where most of the original bubbles or cells remain intact, or "open-cell", where the bubbles have broken but the edges of the bubbles are stiff enough to retain their shape, in extreme cases reticulated foams can be formed. Open-cell foams feel soft and allow air to flow through, so they are comfortable when used in seat cushions or mattresses. Closed-cell foams are used as rigid thermal insulation. High-density microcellular Microcellular plastics, otherwise known as microcellular foam, is a form of manufactured plastic fabricated to contain billions of tiny bubbles less than 50 microns wide (typically 0.1–100 micrometers). It is formed by dissolving gas under high p ...
foams can be formed without the addition of blowing agents by mechanically frothing the polyol prior to use. These are tough elastomeric materials used in covering car steering wheel
A steering wheel (also called a driving wheel (UK), a hand wheel, or simply wheel) is a type of steering control in vehicles.
Steering wheels are used in most modern land vehicles, including all mass-production automobiles, buses, light and ...
s or shoe soles.
The properties of a polyurethane are greatly influenced by the types of isocyanates and polyols used to make it. Long, flexible segments, contributed by the polyol, give soft, elastic polymer. High amounts of crosslinking Cross-linking may refer to
*Cross-link
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers ca ...
give tough or rigid polymers. Long chains and low crosslinking give a polymer that is very stretchy, short chains with many crosslinks produce a hard polymer while long chains and intermediate crosslinking give a polymer useful for making foam. The choices available for the isocyanates and polyols, in addition to other additives and processing conditions allow polyurethanes to have the very wide range of properties that make them such widely used polymers.
Raw materials
The main ingredients to make a polyurethane are di- and tri- isocyanates and polyols. Other materials are added to aid processing the polymer or to modify the properties of the polymer.Isocyanates
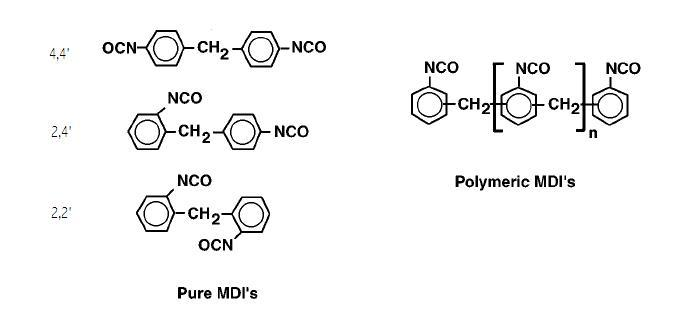
Isocyanates used to make polyurethane have two or more isocyanate groups on each molecule. The most commonly used isocyanates are the aromatic diisocyanates, toluene diisocyanate (TDI) and methylene diphenyl diisocyanate, (MDI). These aromatic isocyanates are more reactive than aliphatic isocyanates. TDI and MDI are generally less expensive and more reactive than other isocyanates. Industrial grade TDI and MDI are mixtures of isomers and MDI often contains polymeric materials. They are used to make flexible foam (for example slabstock foam for mattresses or molded foams for car seats), rigid foam (for example insulating foam in refrigerators) elastomers (shoe soles, for example), and so on. The isocyanates may be modified by partially reacting them with polyols or introducing some other materials to reduce volatility (and hence toxicity) of the isocyanates, decrease their freezing points to make handling easier or to improve the properties of the final polymers.Polyols
Polyols are polymers in their own right and have on average two or more hydroxyl groups per molecule. They can be converted to polyether polyols co-polymerizing ethylene oxide and propylene oxide with a suitable polyol precursor. Polyester polyols are made by the polycondensation of multifunctionalcarboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
s and polyhydroxyl compounds. They can be further classified according to their end use. Higher molecular weight polyols (molecular weights from 2,000 to 10,000) are used to make more flexible polyurethanes while lower molecular weight polyols make more rigid products.
Polyols for flexible applications use low functionality initiators such as dipropylene glycol (''f'' = 2), glycerine (''f'' = 3), or a sorbitol/water solution (''f'' = 2.75). Polyols for rigid applications use high functionality initiators such as sucrose
Sucrose, a disaccharide, is a sugar composed of glucose and fructose subunits. It is produced naturally in plants and is the main constituent of white sugar. It has the molecular formula .
For human consumption, sucrose is extracted and refined ...
(''f'' = 8), sorbitol
Sorbitol (), less commonly known as glucitol (), is a sugar alcohol with a sweet taste which the human body metabolizes slowly. It can be obtained by reduction of glucose, which changes the converted aldehyde group (−CHO) to a primary alcohol g ...
(''f'' = 6), toluenediamine (''f'' = 4), and Mannich base A Mannich base is a beta-amino-ketone, which is formed in the reaction of an amine, formaldehyde (or an aldehyde) and a carbon acid. The Mannich base is an endproduct in the Mannich reaction, which is nucleophilic addition reaction of a non-enoliz ...
s (''f'' = 4). Propylene oxide and/or ethylene oxide is added to the initiators until the desired molecular weight is achieved. The order of addition and the amounts of each oxide affect many polyol properties, such as compatibility, water-solubility, and reactivity. Polyols made with only propylene oxide are terminated with secondary hydroxyl groups and are less reactive than polyols capped with ethylene oxide, which contain primary hydroxyl groups. Incorporating carbon dioxide into the polyol structure is being researched by multiple companies.
Graft polyols (also called filled polyols or polymer polyols) contain finely dispersed styrene–acrylonitrile, acrylonitrile, or polyurea (PHD) polymer solids chemically grafted to a high molecular weight polyether backbone. They are used to increase the load-bearing properties of low-density high-resiliency (HR) foam, as well as add toughness to microcellular foams and cast elastomers. Initiators such as ethylenediamine
Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately ...
and triethanolamine are used to make low molecular weight rigid foam polyols that have built-in catalytic activity due to the presence of nitrogen atoms in the backbone. A special class of polyether polyols, poly(tetramethylene ether) glycols, which are made by polymerizing tetrahydrofuran, are used in high performance coating, wetting and elastomer applications.
Conventional polyester polyols are based on virgin raw materials and are manufactured by the direct polyesterification of high-purity diacids and glycols, such as adipic acid and 1,4-butanediol. Polyester polyols are usually more expensive and more viscous than polyether polyols, but they make polyurethanes with better solvent, abrasion, and cut resistance. Other polyester polyols are based on reclaimed raw materials. They are manufactured by transesterification (glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvate (). The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH ...
) of recycled poly(ethyleneterephthalate) (PET) or dimethylterephthalate
Dimethyl terephthalate (DMT) is an organic compound with the formula C6H4(COOCH3)2. It is the diester formed from terephthalic acid and methanol. It is a white solid that melts to give a distillable colourless liquid.Richard J. Sheehan "Tereph ...
(DMT) distillation bottoms with glycols such as diethylene glycol. These low molecular weight, aromatic polyester polyols are used in rigid foam, and bring low cost and excellent flammability characteristics to polyisocyanurate
Polyisocyanurate (), also referred to as PIR, polyiso, or ISO, is a thermoset plastic typically produced as a foam and used as rigid thermal insulation. The starting materials are similar to those used in polyurethane (PUR) except that the prop ...
(PIR) boardstock and polyurethane spray foam insulation.
Specialty polyols include polycarbonate
Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, tough materials, and some grades are optically transparent. They are easily work ...
polyols, polycaprolactone polyols, polybutadiene
Polybutadiene utadiene rubber BRis a synthetic rubber. Polybutadiene rubber is a polymer formed from the polymerization of the monomer 1,3-butadiene. Polybutadiene has a high resistance to wear and is used especially in the manufacture of tir ...
polyols, and polysulfide polyols. The materials are used in elastomer, sealant, and adhesive applications that require superior weatherability, and resistance to chemical and environmental attack. Natural oil polyols Natural oil polyols, also known as NOPs or biopolyols, are polyols derived from vegetable oils by several different techniques. The primary use for these materials is in the production of polyurethanes. Most NOPs qualify as biobased products, as d ...
derived from castor oil and other vegetable oils are used to make elastomers, flexible bunstock, and flexible molded foam.
Co-polymerizing chlorotrifluoroethylene or tetrafluoroethylene with vinyl ethers containing hydroxyalkyl vinyl ether produces fluorinated (FEVE) polyols. Two-component fluorinated polyurethanes prepared by reacting FEVE fluorinated polyols with polyisocyanate have been used to make ambient cure paints and coatings. Since fluorinated polyurethanes contain a high percentage of fluorine–carbon bonds, which are the strongest bonds among all chemical bonds, fluorinated polyurethanes exhibit resistance to UV, acids, alkali, salts, chemicals, solvents, weathering, corrosion, fungi and microbial attack. These have been used for high performance coatings and paints.
Phosphorus-containing polyols are available that become chemically bonded to the polyurethane matrix for the use as flame retardants. This covalent linkage prevents migration and leaching of the organophosphorus compound.
Bio-derived materials
Interest insustainable
Specific definitions of sustainability are difficult to agree on and have varied in the literature and over time. The concept of sustainability can be used to guide decisions at the global, national, and individual levels (e.g. sustainable livin ...
"green" products raised interest in polyols derived from vegetable oils.
Various oils used in the preparation polyols for polyurethanes include soybean, cotton seed, neem seed, and castor. Vegetable oils are functionalized by various ways and modified to polyetheramide, polyethers, alkyds, etc. Renewable sources used to prepare polyols may be dimer fatty acids or fatty acids. Some biobased and isocyanate-free polyurethanes exploit the reaction between polyamines and cyclic carbonates to produce polyhydroxurethanes.
Chain extenders and cross linkers
Chain extenders (''f'' = 2) and cross linkers (''f'' ≥ 3) are low molecular weight hydroxyl and amine terminated compounds that play an important role in the polymer morphology of polyurethane fibers, elastomers, adhesives, and certain integral skin and microcellular foams. The elastomeric properties of these materials are derived from the phase separation of the hard and soft copolymer segments of the polymer, such that the urethane hard segment domains serve as cross-links between the amorphous polyether (or polyester) soft segment domains. This phase separation occurs because the mainly nonpolar, low melting soft segments are incompatible with the polar, high melting hard segments. The soft segments, which are formed from high molecular weight polyols, are mobile and are normally present in coiled formation, while the hard segments, which are formed from the isocyanate and chain extenders, are stiff and immobile. Because the hard segments are covalently coupled to the soft segments, they inhibit plastic flow of the polymer chains, thus creating elastomeric resiliency. Upon mechanical deformation, a portion of the soft segments are stressed by uncoiling, and the hard segments become aligned in the stress direction. This reorientation of the hard segments and consequent powerful hydrogen bonding contributes to high tensile strength, elongation, and tear resistance values. The choice of chain extender also determines flexural, heat, and chemical resistance properties. The most important chain extenders are ethylene glycol,1,4-butanediol
1,4-Butanediol, colloquially known as BD or BDO, is a primary alcohol, and an organic compound, with the formula HOCH2CH2CH2CH2OH. It is a colorless viscous liquid. It is one of four stable isomers of butanediol.
Synthesis
In industrial sy ...
(1,4-BDO or BDO), 1,6-hexanediol
1,6-Hexanediol is an organic compound with the formula (CH2CH2CH2OH)2. It is a colorless water-soluble solid.
Production
1,6-Hexanediol is prepared by the hydrogenation of adipic acid or its esters. Laboratory preparation could be achieved by r ...
, cyclohexane dimethanol and hydroquinone bis(2-hydroxyethyl) ether
Hydroquinone, also known as benzene-1,4-diol or quinol, is an aromatic organic compound that is a type of Phenols, phenol, a derivative of benzene, having the chemical formula C6H4(OH)2. It has two hydroxyl groups covalent bond, bonded to a benze ...
(HQEE). All of these glycols form polyurethanes that phase separate well and form well defined hard segment domains, and are melt processable. They are all suitable for thermoplastic polyurethanes with the exception of ethylene glycol, since its derived bis-phenyl urethane undergoes unfavorable degradation at high hard segment levels. Diethanolamine and triethanolamine are used in flex molded foams to build firmness and add catalytic activity. Diethyltoluenediamine is used extensively in RIM, and in polyurethane and polyurea elastomer formulations.
Catalysts
Polyurethane catalysts can be classified into two broad categories, basic and acidic amine. Tertiary amine catalysts function by enhancing the nucleophilicity of the diol component. Alkyl tin carboxylates, oxides and mercaptides oxides function as mild Lewis acids in accelerating the formation of polyurethane. As bases, traditional amine catalysts