|
DABCO
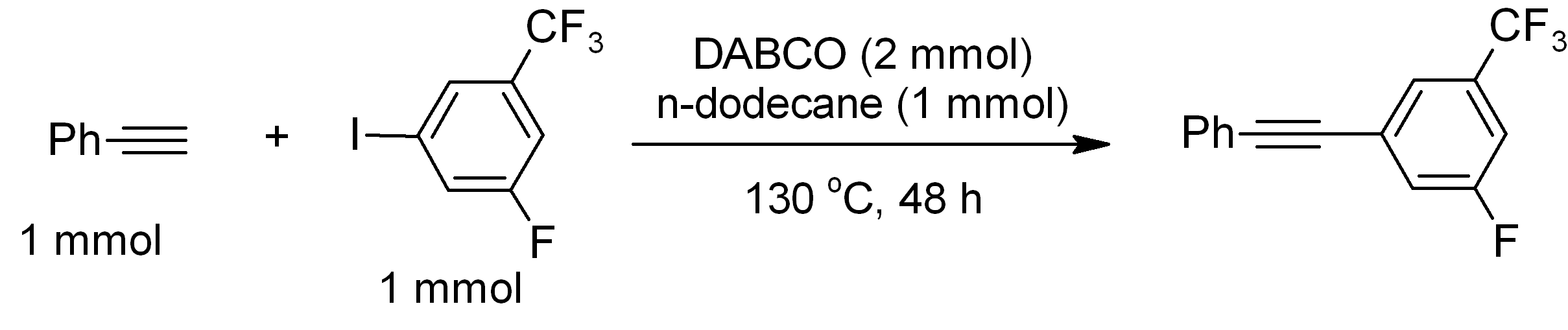
DABCO (1,4-diazabicyclo .2.2ctane), also known as triethylenediamine or TEDA, is a bicyclic organic compound with the formula N2(C2H4)3. This colorless solid is a highly nucleophilic tertiary amine base, which is used as a catalyst and reagent in polymerization and organic synthesis. It is similar in structure to quinuclidine, but the latter has one of the nitrogen atoms replaced by a carbon atom. Reactions The p''K''a of DABCOsup>+ (the protonated derivative) is 8.8, which is almost the same as ordinary alkylamines. The nucleophilicity of the amine is high because the amine centers are unhindered. It is sufficiently basic to promote C–C coupling of terminal acetylenes, for example, phenylacetylene couples with electron-deficient iodoarenes. : Catalyst DABCO is used as a base-catalyst for: *formation of polyurethane from alcohol and isocyanate functionalized monomers and pre-polymers. * Baylis-Hillman reactions of aldehydes and unsaturated ketones and aldehydes. : ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DABCO Sonogashira
DABCO (1,4-diazabicyclo .2.2ctane), also known as triethylenediamine or TEDA, is a bicyclic organic compound with the formula N2(C2H4)3. This colorless solid is a highly nucleophilic tertiary amine base, which is used as a catalyst and reagent in polymerization and organic synthesis. It is similar in structure to quinuclidine, but the latter has one of the nitrogen atoms replaced by a carbon atom. Reactions The p''K''a of DABCOsup>+ (the protonated derivative) is 8.8, which is almost the same as ordinary alkylamines. The nucleophilicity of the amine is high because the amine centers are unhindered. It is sufficiently basic to promote C–C coupling of terminal acetylenes, for example, phenylacetylene couples with electron-deficient iodoarenes. : Catalyst DABCO is used as a base-catalyst for: *formation of polyurethane from alcohol and isocyanate functionalized monomers and pre-polymers. * Baylis-Hillman reactions of aldehydes and unsaturated ketones and aldehydes. : Lewis bas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bicyclic Molecule
In chemistry, a bicyclic molecule () is a molecule that features two joined rings. Bicyclic structures occur widely, for example in many biologically important molecules like α-thujene and camphor. A bicyclic compound can be carbocyclic (all of the ring atoms are carbons), or heterocyclic (the rings' atoms consist of at least two elements), like DABCO. Moreover, the two rings can both be aliphatic (''e.g.'' decalin and norbornane), or can be aromatic (''e.g.'' naphthalene), or a combination of aliphatic and aromatic (''e.g.'' tetralin). Three modes of ring junction are possible for a bicyclic compound: * In spirocyclic compounds, the two rings share only one single atom, the spiro atom, which is usually a quaternary carbon. An example of a spirocyclic compound is the photochromic switch spiropyran. * In fused/condensed bicyclic compounds, two rings share two adjacent atoms. In other words, the rings share one covalent bond, ''i.e.'' the so-called bridgehead atoms are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyurethane
Polyurethane (; often abbreviated PUR and PU) refers to a class of polymers composed of organic chemistry, organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane is produced from a wide range of starting materials. This chemical variety produces polyurethanes with different chemical structures leading to many List of polyurethane applications, different applications. These include rigid and flexible foams, varnishes and coatings, adhesives, Potting (electronics), electrical potting compounds, and fibers such as spandex and Polyurethane laminate, PUL. Foams are the largest application accounting for 67% of all polyurethane produced in 2016. A polyurethane is typically produced by reacting an isocyanate with a polyol. Since a polyurethane contains two types of monomers, which polymerize one after the other, they are classed as Copolymer#Alternating copolymers, alternating copolymers. Both the isocy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Baylis Hilman Reaction Scheme
Baylis may refer to: Places *Baylis, Illinois, a village in Pike County, Illinois, United States * Baylis, Slough, a place in the English county of Berkshire * Baylis, the seat of Alexander Wedderburn, 1st Earl of Rosslyn near Salt Hill, Windsor where he died in 1805 * Baylis Road, a road in Lambeth, London, England *Baylis & Harding, the handwash company based in Redditch, England *Baylis Street, one of the main shopping streets in Wagga Wagga, New South Wales, Australia *Baylis Court School, a girls' school in Slough, Berkshire, England Other uses *Baylis (surname) * Baylis–Hillman reaction, a reaction of an aldehyde and an α,β-unsaturated electron-withdrawing group catalyzed by DABCO (1,4-diazabicyclo .2.2ctane) to give an allylic alcohol *Aza-Baylis–Hillman reaction, the reaction of an α,β-unsaturated carbonyl compound with an imine in the presence of a nucleophile See also *Bayless Bayless is a surname. Notable people with the surname include: * Becky Bayless (born ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinuclidine
Quinuclidine is an organic compound and a bicyclic amine and used as a catalyst and a chemical building block. It is a strong base with p''K''a of the conjugate acid of 11.0.{{cite journal , title=Azatriquinanes: Synthesis, Structure, and Reactivity , author1=Hext, N. M. , author2=Hansen, J. , author3=Blake, A. J. , author4=Hibbs, D. E. , author5=Hursthouse, M. B. , author6=Shishkin, O. V. , author7=Mascal, M. , journal=J. Org. Chem. , year=1998 , volume=63 , issue=17 , pages=6016–6020 , doi=10.1021/jo980788s , pmid=11672206 It can be prepared by reduction of quinuclidone. In alkane solvents quinuclidine is a Lewis base that forms adducts with a variety of Lewis acids. The compound is structurally related to DABCO, in which the other bridgehead is also nitrogen, and to tropane, which has a slightly different carbon frame. Quinuclidine is found as a structural component of some biomolecules including quinine Quinine is a medication used to treat malaria and babesiosis. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flexplay
Flexplay is a trademark for a discontinued DVD-compatible optical video disc format with a time-limited (usually 48-hour) playback. They are often described as "self-destructing", although the disc merely turns black or dark red and does not physically disintegrate. The technology launched in August 2003 as a joint-venture with Disney's Buena Vista Home Entertainment under the name eZ-D. The Flexplay concept was invented by two professors, Yannis Bakos and Erik Brynjolfsson, who founded Flexplay Technologies in 1999. The technology was developed by Flexplay Technologies and General Electric. Origins The technology was originally intended as an alternative means for the short-term rental of newly released movies. Since the disc is capable of being used in any standard DVD player, the manufacturers hoped that it would succeed where other time-limited DVD technologies, such as DIVX, failed. Test marketing of eZ-D discs began in August 2003, but was canceled early when consumer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%–6% by weight) in water for consumer use, and in higher concentrations for industrial use. Concentrated hydrogen peroxide, or " high-test peroxide", decomposes explosively when heated and has been used as a propellant in rocketry. Hydrogen peroxide is a reactive oxygen species and the simplest peroxide, a compound having an oxygen–oxygen single bond. It decomposes slowly when exposed to light, and rapidly in the presence of organic or reactive compounds. It is typically stored with a stabilizer in a weakly acidic solution in a dark bottle to block light. Hydrogen peroxide is found in biological systems including the human body. Enzymes that use or decompose hydrogen peroxide are classified as peroxidases. Properties The boiling ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Dioxide
Sulfur dioxide ( IUPAC-recommended spelling) or sulphur dioxide (traditional Commonwealth English) is the chemical compound with the formula . It is a toxic gas responsible for the odor of burnt matches. It is released naturally by volcanic activity and is produced as a by-product of copper extraction and the burning of sulfur- bearing fossil fuels. Structure and bonding SO2 is a bent molecule with ''C''2v symmetry point group. A valence bond theory approach considering just ''s'' and ''p'' orbitals would describe the bonding in terms of resonance between two resonance structures. The sulfur–oxygen bond has a bond order of 1.5. There is support for this simple approach that does not invoke ''d'' orbital participation. In terms of electron-counting formalism, the sulfur atom has an oxidation state of +4 and a formal charge of +1. Occurrence Sulfur dioxide is found on Earth and exists in very small concentrations and in the atmosphere at about 1 ppm. On other p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta-lactamase Inhibitors
Beta-lactamases, (β-lactamases) are enzymes () produced by bacteria that provide multi-resistance to beta-lactam antibiotics such as penicillins, cephalosporins, cephamycins, monobactams and carbapenems ( ertapenem), although carbapenems are relatively resistant to beta-lactamase. Beta-lactamase provides antibiotic resistance by breaking the antibiotics' structure. These antibiotics all have a common element in their molecular structure: a four-atom ring known as a beta-lactam (β-lactam) ring. Through hydrolysis, the enzyme lactamase breaks the β-lactam ring open, deactivating the molecule's antibacterial properties. Beta-lactam antibiotics are typically used to target a broad spectrum of gram-positive and gram-negative bacteria. Beta-lactamases produced by gram-negative bacteria are usually secreted, especially when antibiotics are present in the environment. Structure The structure of a ''Streptomyces'' serine β-lactamase (SBLs) is given by . The alpha-beta fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of The American Chemical Society
The ''Journal of the American Chemical Society'' is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ''Journal of Analytical and Applied Chemistry'' (July 1893) and the ''American Chemical Journal'' (January 1914). It covers all fields of chemistry. Since 2021, the editor-in-chief is Erick M. Carreira ( ETH Zurich). In 2014, the journal moved to a hybrid open access publishing model. Abstracting and indexing The journal is abstracted and indexed in Chemical Abstracts Service, Scopus, EBSCO databases, ProQuest databases, Index Medicus/ MEDLINE/ PubMed, and the Science Citation Index Expanded. According to the '' Journal Citation Reports'', the journal has a 2021 impact factor of 16.383. Editors-in-chief The following people are or have been editor-in-chief: * 1879–1880 – Hermann Endemann * 1880–1881 – Gideon E. Moore * 1881–1882 – Hermann ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewis Acids And Bases
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. For example, NH3 is a Lewis base, because it can donate its lone pair of electrons. Trimethylborane (Me3B) is a Lewis acid as it is capable of accepting a lone pair. In a Lewis adduct, the Lewis acid and base share an electron pair furnished by the Lewis base, forming a dative bond. In the context of a specific chemical reaction between NH3 and Me3B, a lone pair from NH3 will form a dative bond with the empty orbital of Me3B to form an adduct NH3•BMe3. The terminology refers to the contributions of Gilbert N. Lewis. From p. 142: "We are inclined to think of substances as poss ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |