|
Chlorotrifluoroethylene
Chlorotrifluoroethylene (CTFE) is a chlorofluorocarbon with chemical formula CFCl=CF2. It is commonly used as a refrigerant in cryogenic applications. CTFE has a carbon-carbon double bond and so can be polymerized to form polychlorotrifluoroethylene or copolymerized to produce the plastic ECTFE. PCTFE has the trade name Neoflon PCTFE from Daikin Industries in Japan, and it used to be produced under the trade name Kel-F from 3M Corporation in Minnesota. Production and reactions Chlorotrifluoroethylene is produced commercially by the dechlorination of 1,1,2-trichloro-1,2,2-trifluoroethane with zinc: :CFCl2-CF2Cl + Zn → CClF=CF2 + ZnCl2 In 2012, an estimated 1–10 million pounds were produced commercially in the United States. Addition of iodine monochloride to chlorotrifluoroethylene gives iododichlorotrifluoroethane: : The latter is a precursor to hexafluorobutadiene. Thermal dimerization of chlorotrifluoroethylene gives 1,2-dichloro-1,2,3,3,4,4-hexafluorocyclobutane ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polychlorotrifluoroethylene
Polychlorotrifluoroethylene (PCTFE or PTFCE) is a thermoplastic chlorofluoropolymer with the molecular formula , where ''n'' is the number of monomer units in the polymer molecule. It is similar to polytetrafluoroethene (PTFE), except that it is a homopolymer of the monomer chlorotrifluoroethylene (CTFE) instead of tetrafluoroethene. It has the lowest water vapor transmission rate of any plastic. History It was discovered in 1934 by Fritz Schloffer and Otto Scherer who worked at IG Farben Company, Germany. Trade names After World War II, PCTFE was commercialized under the trade name of Kel-F 81 by M. W. Kellogg Company in early 1950s. The name "Kel-F" was derived from "Kellogg" and "fluoropolymer", which also represents other fluoropolymers like the copolymer poly(chlorotrifluoroethylene-co-vinylidene fluoride) (Kel-F 800). These were acquired by 3M Company in 1957. 3M discontinued manufacturing of Kel-F by 1996. PCTFE resin is now manufactured under different trade na ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ECTFE
ECTFE (ethylene-chlorotrifluoroethylene) is an alternating copolymer of ethylene and chlorotrifluoroethylene. It is a semi-crystalline fluoropolymer (a partly fluorinated polymer), with chemical corrosion resistance properties. Physical and chemical properties ECTFE (ethylene chlorotrifluoroethylene) is a polymer known for its chemical resistance, making it suitable for various industrial applications. It is resistant to acids at high concentrations/temperatures, caustic media, oxidizing agents, and many solvents, similar to PTFE (polytetrafluoroethylene). One of the key properties of ECTFE is its permeation resistance to large molecules, which is generally slow and not significant in practical applications. Small molecules, however, may permeate through the polymer matrix. In lining or coating applications using ECTFE, permeability of certain small molecules determines the lifetime of anti-corrosion protection. Small molecules such as H2O, O2, Cl2, H2S, HCl, HF, HBr, N ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromotrifluoroethylene
Bromotrifluoroethylene (BTFE) is a halogenated ethylene derivative with the chemical formula . It is a highly flammable colourless gas with a musty odour resembling phosgene. It can polymerise spontaneously.Arthur J. Elliott''Bromotrifluoroethylene''in Kirk-Othmer Encyclopedia of Chemical Technology Preparation Bromotrifluoroethylene can be prepared from chlorotrifluoroethylene with high yields: : : It was first prepared by the Belgian chemist Frédéric Swarts in 1899.''Industrial Polymers and Radiation: Proceedings of the Symposium Held at Sardar Patel University'', Vallabh Vidyanagar, Gujarat, February 12–14, 1979. Reactions and uses Bromotrifluoroethylene forms metal complexes with substituted phosphine compounds and platinum(II).V.A. Mukhedkar, B.J. Kavathekar, A.J. Mukhedkar''Reactions of metal complexes Rearrangement reactions of bromotrifluoroethylene-bis(substituted phosphine)-platinum (II)'' Journal of Inorganic and Nuclear Chemistry, Volume 37, Issue 2, February 1975, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexafluorocyclobutene
Hexafluorocyclobutene is the organofluorine compound with the formula (CF2)2(CF)2. A colorless gas, it is a precursor to a variety of compounds, including squaric acid. Hexafluorocyclobutene is prepared in two steps from chlorotrifluoroethylene. The thermal dimerization gives 1,2-dichloro-1,2,3,3,4,4-hexafluorocyclobutane. Dechlorination of the latter gives hexafluorocyclobutene: :C4F6Cl2 + Zn → C4F6 + ZnCl2 Safety Reminiscent of perfluoroisobutene, hexafluorocyclobutene is quite toxic with an LD = 6000 mg/min/m−3 (mice).{{cite book , doi=10.1016/B978-008043405-6/50040-2, chapter=Highly-toxic fluorine compounds, title=Fluorine Chemistry at the Millennium, year=2000, last1=Timperley, first1=Christopher M., pages=499–538, isbn=9780080434056 See also *Hexafluoro-2-butyne *Hexafluorobutadiene Hexafluorobutadiene is an organofluorine compound Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–flu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexafluorobutadiene
Hexafluorobutadiene is an organofluorine compound Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, ... with the formula (CF2=CF)2. A colorless gas, it has attracted attention as an etchant in microelectronics. It is the perfluoroanalogue of butadiene. Preparation It can be prepared by coupling of fluorinated C2 precursors. Addition of iodine monochloride to chlorotrifluoroethylene gives iododichlorotrifluoroethane that can be coupled in the presence of mercury to give 1,2,3,4-tetrchlorohexafluorobutane: : Zn-induced dechlorination of this tetrachloride gives the desired perfluorinated diene: : Reactions The diene can be rehalogenated, e.g. with bromine upon UV irradiation: : Hexafluorobutadiene dimerizes via a [2+2] process at 150 °C to give perfluorinated divinylcyclobutanes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
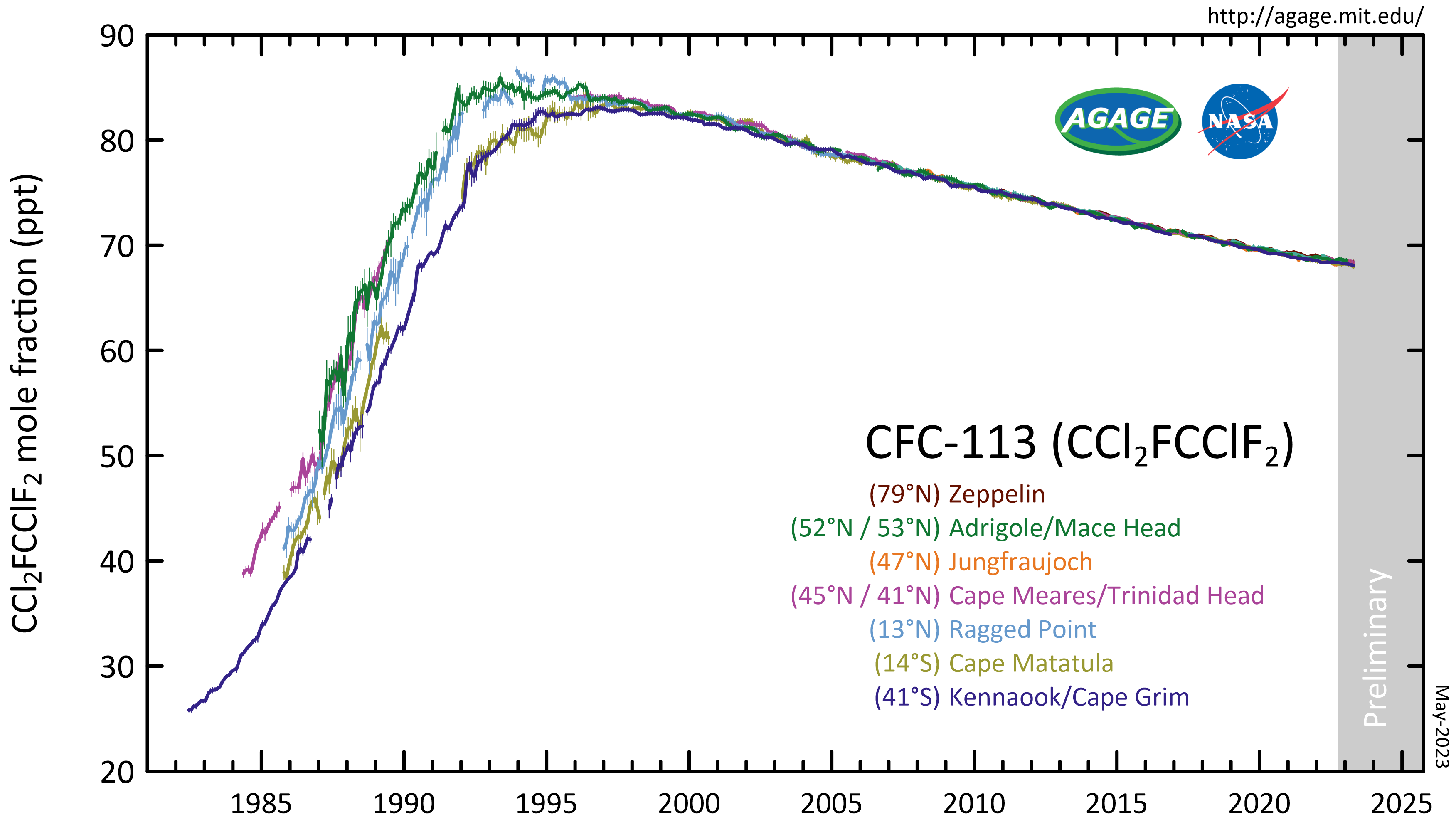
1,1,2-trichloro-1,2,2-trifluoroethane
1,1,2-Trichloro-1,2,2-trifluoroethane, also called trichlorotrifluoroethane (often abbreviated as TCTFE) or CFC-113, is a chlorofluorocarbon. It has the formula . This colorless, volatile liquid is a versatile solvent. Production CFC-113 can be prepared from hexachloroethane and hydrofluoric acid: : This reaction may require catalysts such as antimony, chromium, iron and alumina at high temperatures. Another synthesis method uses HF on tetrachloroethylene instead. Atmospheric reactions CFC-113 is a very unreactive chlorofluorocarbon. It may remain in the atmosphere up to 90 years, sufficiently long that it will cycle out of the troposphere and into the stratosphere. In the stratosphere, CFC-113 can be broken up by ultraviolet radiation (UV, sunlight in the 190-225 nm range), generating chlorine radicals (Cl•), which initiate degradation of ozone requiring only a few minutes: : : This reaction is followed by: : The process regenerates Cl• to destroy more . The Cl• wil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Daikin Industries
is a Japanese multinational conglomerate company headquartered in Osaka. Daikin is the world's largest air conditioner manufacturer. History Daikin Industries Ltd was founded in 1924 as by Akira Yamada. In 1953, Daiflon or polychlorotrifluoroethylene was developed. In 1963 the company was renamed and developed Neoflon. In 1982 it was renamed to the current Daikin Industries Ltd. Daikin entered the North American air conditioning market in 2004. In 2006, Daikin Industries acquired McQuay International, a Minneapolis, Minnesota–based global corporation that designs, manufacturers and sells commercial, industrial and institutional heating, ventilation and air conditioning (HVAC) products. In 2008, McQuay International was rebranded as Daikin-McQuay as Daikin began implementing many of its technologies (including the Daikin Inverter Compressor) and manufacturing processes into McQuay equipment and factories. However, in November 2013, the Daikin-McQuay group was agai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
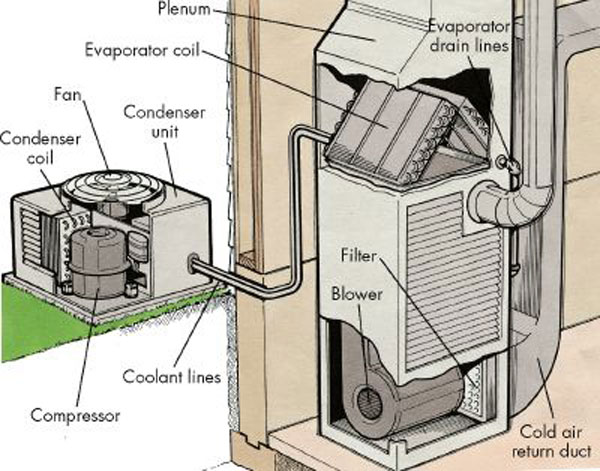
Refrigerants
A refrigerant is a working fluid used in the cooling, heating, or reverse cooling/heating cycles of air conditioning systems and heat pumps, where they undergo a repeated phase transition from a liquid to a gas and back again. Refrigerants are heavily regulated because of their toxicity and flammability, as well as the contribution of CFC and HCFC refrigerants to ozone depletion and the contribution of HFC refrigerants to climate change. Refrigerants are used in a direct expansion (DX) circulating system to transfer energy from one environment to another, typically from inside a building to outside or vice versa. These can be air conditioner cooling only systems, cooling & heating reverse DX systems, or heat pump and heating only DX cycles. Refrigerants are controlled substances that are classified by several international safety regulations and, depending on their classification, may only be handled by qualified engineers due to extreme pressure, temperature, flammability, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heating, Ventilation, And Air Conditioning
Heating, ventilation, and air conditioning (HVAC ) is the use of various technologies to control the temperature, humidity, and purity of the air in an enclosed space. Its goal is to provide thermal comfort and acceptable indoor air quality. HVAC system design is a subdiscipline of mechanical engineering, based on the principles of thermodynamics, fluid mechanics, and heat transfer. "Refrigeration" is sometimes added to the field's abbreviation as HVAC&R or HVACR, or "ventilation" is dropped, as in HACR (as in the designation of HACR-rated circuit breakers). HVAC is an important part of residential structures such as single family homes, apartment buildings, hotels, and senior living facilities; medium to large industrial and office buildings such as skyscrapers and hospitals; vehicles such as cars, trains, airplanes, ships and submarines; and in marine environments, where safe and Sick building syndrome, healthy building conditions are regulated with respect to temperature and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinyl Acetate
Vinyl acetate is an organic compound with the Chemical formula, formula CH3CO2CH=CH2. This colorless liquid is the precursor to polyvinyl acetate, ethylene-vinyl acetate copolymers, polyvinyl alcohol, and other important industrial polymers. Production The worldwide production capacity of vinyl acetate was estimated at 6,969,000 tonnes/year in 2007, with most capacity concentrated in the United States (1,585,000 all in Texas), China (1,261,000), Japan (725,000) and Taiwan (650,000). The average list price for 2008 was US$1600/tonne. Celanese is the largest producer (ca 25% of the worldwide capacity), while other significant producers include China Petrochemical Corporation (7%), Chang Chun Group (6%), and LyondellBasell (5%). Vinyl acetate is mainly (80%) polymerized, and the resulting polymer is hydrolyzed to give polyvinvyl alcohol. Preparation Vinyl acetate is the acetate ester of vinyl alcohol. Since vinyl alcohol is highly unstable (with respect to acetaldehyde), the prepara ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine Monochloride
Iodine monochloride is an interhalogen compound with the formula . It is a red-brown chemical compound that melts near room temperature. Because of the difference in the electronegativity of iodine and chlorine, this molecule is highly polar and behaves as a source of I+. Discovered in 1814 by Gay-Lussac, iodine monochloride is the first interhalogen compound discovered. Preparation Iodine monochloride is produced simply by combining the halogens in a 1:1 molar ratio, according to the equation : When chlorine gas is passed through iodine crystals, one observes the brown vapor of iodine monochloride. Dark brown iodine monochloride liquid is collected. Excess chlorine converts iodine monochloride into iodine trichloride in a reversible reaction: : Polymorphs has two polymorphs; α-ICl, which exists as black needles (red by transmitted light) with a melting point of 27.2 °C, and β-ICl, which exists as black platelets (red-brown by transmitted light) with a melt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |