
Distillation, also classical distillation, is the process of
separating the component substances of a liquid
mixture
In chemistry, a mixture is a material made up of two or more different chemical substances which can be separated by physical method. It is an impure substance made up of 2 or more elements or compounds mechanically mixed together in any proporti ...
of two or more chemically discrete substances; the separation process is realized by way of the selective
boiling
Boiling or ebullition is the rapid phase transition from liquid to gas or vapor, vapour; the reverse of boiling is condensation. Boiling occurs when a liquid is heated to its boiling point, so that the vapour pressure of the liquid is equal to ...
of the mixture and the
condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor ...
of the vapors in a
still
A still is an apparatus used to distillation, distill liquid mixtures by heating to selectively Boiling, boil and then cooling to Condensation, condense the vapor. A still uses the same concepts as a basic Distillation#Laboratory_procedures, ...
.
Distillation can operate over a wide range of pressures from 0.14
bar (e.g.,
ethylbenzene/
styrene
Styrene is an organic compound with the chemical formula C6H5CH=CH2. Its structure consists of a vinyl group as substituent on benzene. Styrene is a colorless, oily liquid, although aged samples can appear yellowish. The compound evaporates easi ...
) to nearly 21 bar (e.g.,
propylene
Propylene, also known as propene, is an unsaturated organic compound with the chemical formula . It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petroleum-like o ...
/
propane
Propane () is a three-carbon chain alkane with the molecular formula . It is a gas at standard temperature and pressure, but becomes liquid when compressed for transportation and storage. A by-product of natural gas processing and petroleum ref ...
) and is capable of separating feeds with high volumetric flowrates and various components that cover a range of relative volatilities from only 1.17 (
o-xylene/
m-xylene) to 81.2 (water/
ethylene glycol). Distillation provides a convenient and time-tested solution to separate a diversity of chemicals in a continuous manner with high purity. However, distillation has an enormous environmental footprint, resulting in the consumption of approximately 25% of all industrial energy use. The key issue is that distillation operates based on phase changes, and this separation mechanism requires vast energy inputs.
Dry distillation
Dry distillation is the heating of solid materials to produce gaseous products (which may condense into liquids or solids). The method may involve pyrolysis or thermolysis, or it may not (for instance, a simple mixture of ice and glass could be ...
(
thermolysis and
pyrolysis
Pyrolysis is a process involving the Bond cleavage, separation of covalent bonds in organic matter by thermal decomposition within an Chemically inert, inert environment without oxygen. Etymology
The word ''pyrolysis'' is coined from the Gree ...
) is the heating of solid materials to produce gases that condense either into
fluid
In physics, a fluid is a liquid, gas, or other material that may continuously motion, move and Deformation (physics), deform (''flow'') under an applied shear stress, or external force. They have zero shear modulus, or, in simpler terms, are M ...
products or into solid products. The term ''dry distillation'' includes the separation processes of
destructive distillation
Destructive distillation is a chemical process in which decomposition of unprocessed material is achieved by heating it to a high temperature; the term generally applies to processing of organic material in the absence of air or in the presence o ...
and of
chemical cracking, breaking down large
hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
molecules into smaller hydrocarbon molecules. Moreover, a partial distillation results in partial separations of the mixture's components, which process yields nearly-pure components; partial distillation also realizes partial separations of the mixture to increase the concentrations of selected components. In either method, the separation process of distillation exploits the differences in the
relative volatility
Relative volatility is a measure comparing the vapor pressures of the components in a liquid mixture of chemicals. This quantity is widely used in designing large industrial distillation processes. In effect, it indicates the ease or difficulty of ...
of the component substances of the heated mixture.
In the industrial applications of classical distillation, the term ''distillation'' is used as a
unit of operation that identifies and denotes a process of physical separation, not a
chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
; thus an industrial installation that produces
distilled beverages
Liquor ( , sometimes hard liquor), spirits, distilled spirits, or spiritous liquor are alcoholic drinks produced by the distillation of grains, fruits, vegetables, or sugar that have already gone through alcoholic fermentation. While the ...
, is a distillery of
alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
. These are some applications of the chemical separation process that is distillation:
* Distilling
fermented
Fermentation is a type of anaerobic metabolism which harnesses the redox potential of the reactants to make adenosine triphosphate (ATP) and organic end products. Organic compound, Organic molecules, such as glucose or other sugars, are Catabo ...
products to yield alcoholic beverages with a high content by volume of
ethyl alcohol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol ...
.
*
Desalination
Desalination is a process that removes mineral components from saline water. More generally, desalination is the removal of salts and minerals from a substance. One example is Soil salinity control, soil desalination. This is important for agric ...
to produce potable water and for medico-industrial applications.
*
Crude oil stabilisation
Crude oil stabilisation (or stabilization) is a partial distillation process that renders crude oil suitable for storage in atmospheric tanks, or of a quality suitable for sales or pipeline transportation. Stabilization is achieved by subjecting ...
, a partial distillation to reduce the vapor pressure of crude oil, which thus is safe to store and to transport, and thereby reduces the volume of atmospheric emissions of volatile
hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
s.
*
Fractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation ...
used in the midstream operations of an
oil refinery
An oil refinery or petroleum refinery is an industrial processes, industrial process Factory, plant where petroleum (crude oil) is transformed and refining, refined into products such as gasoline (petrol), diesel fuel, Bitumen, asphalt base, ...
for producing
fuel
A fuel is any material that can be made to react with other substances so that it releases energy as thermal energy or to be used for work (physics), work. The concept was originally applied solely to those materials capable of releasing chem ...
s and chemical
raw material
A raw material, also known as a feedstock, unprocessed material, or primary commodity, is a basic material that is used to produce goods, finished goods, energy, or intermediate materials/Intermediate goods that are feedstock for future finished ...
s for livestock feed.
* Cryogenic
Air separation
An air separation plant separates Atmosphere of Earth, atmospheric air into its primary components, typically nitrogen and oxygen, and sometimes also argon and other rare inert gases.
The most common method for air separation is fractional distill ...
into the component gases —
oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
,
nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
, and
argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
— for use as
industrial gas
Industrial gases are the gaseous materials that are Manufacturing, manufactured for use in Industrial sector, industry. The principal gases provided are nitrogen, oxygen, carbon dioxide, argon, hydrogen, helium and acetylene, although many other ...
es.
*
Chemical synthesis
Chemical synthesis (chemical combination) is the artificial execution of chemical reactions to obtain one or several products. This occurs by physical and chemical manipulations usually involving one or more reactions. In modern laboratory uses ...
to separate impurities and unreacted materials.
History
Iron Age
Early evidence of distillation was found on
Akkadian tablets dated describing perfumery operations. The tablets provided textual evidence that an early, primitive form of distillation was known to the
Babylonia
Babylonia (; , ) was an Ancient history, ancient Akkadian language, Akkadian-speaking state and cultural area based in the city of Babylon in central-southern Mesopotamia (present-day Iraq and parts of Kuwait, Syria and Iran). It emerged as a ...
ns of ancient
Mesopotamia
Mesopotamia is a historical region of West Asia situated within the Tigris–Euphrates river system, in the northern part of the Fertile Crescent. Today, Mesopotamia is known as present-day Iraq and forms the eastern geographic boundary of ...
.
Classical antiquity
Greek and Roman terminology
According to British chemist T. Fairley, neither the Greeks nor the Romans had any term for the modern concept of distillation. Words like "distill" would have referred to something else, in most cases a part of some process unrelated to what now is known as distillation. In the words of Fairley and German chemical engineer Norbert Kockmann respectively:
According to Dutch chemical historian
Robert J. Forbes, the word ''distillare'' (to drip off) when used by the Romans, e.g.
Seneca and
Pliny the Elder
Gaius Plinius Secundus (AD 23/24 79), known in English as Pliny the Elder ( ), was a Roman Empire, Roman author, Natural history, naturalist, and naval and army commander of the early Roman Empire, and a friend of the Roman emperor, emperor Vesp ...
, was "never used in our sense".
Aristotle
Aristotle
Aristotle (; 384–322 BC) was an Ancient Greek philosophy, Ancient Greek philosopher and polymath. His writings cover a broad range of subjects spanning the natural sciences, philosophy, linguistics, economics, politics, psychology, a ...
knew that water condensing from evaporating seawater is fresh:
Letting seawater evaporate and condense into freshwater cannot be called "distillation" for distillation involves boiling, but the experiment may have been an important step towards distillation.
Alexandrian chemists

Early evidence of distillation has been found related to
alchemists
Alchemy (from the Arabic word , ) is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practised in China, India, the Muslim world, and Europe. In its Western form, alchemy is first ...
working in
Alexandria
Alexandria ( ; ) is the List of cities and towns in Egypt#Largest cities, second largest city in Egypt and the List of coastal settlements of the Mediterranean Sea, largest city on the Mediterranean coast. It lies at the western edge of the Nile ...
in
Roman Egypt
Roman Egypt was an imperial province of the Roman Empire from 30 BC to AD 642. The province encompassed most of modern-day Egypt except for the Sinai. It was bordered by the provinces of Crete and Cyrenaica to the west and Judaea, ...
in the 1st century CE.
Distilled water has been in use since at least , when
Alexander of Aphrodisias
Alexander of Aphrodisias (; AD) was a Peripatetic school, Peripatetic philosopher and the most celebrated of the Ancient Greek Commentaries on Aristotle, commentators on the writings of Aristotle. He was a native of Aphrodisias in Caria and liv ...
described the process.
Work on distilling other liquids continued in early
Byzantine Egypt
Roman Egypt was an imperial province of the Roman Empire from 30 BC to AD 642. The province encompassed most of modern-day Egypt except for the Sinai. It was bordered by the provinces of Crete and Cyrenaica to the west and Judaea, l ...
under
Zosimus of Panopolis in the 3rd century.
Ancient India and China (1–500 CE)
Distillation was practiced in the ancient
Indian subcontinent
The Indian subcontinent is a physiographic region of Asia below the Himalayas which projects into the Indian Ocean between the Bay of Bengal to the east and the Arabian Sea to the west. It is now divided between Bangladesh, India, and Pakista ...
, which is evident from baked clay
retort
In a chemistry laboratory, a retort is a device used for distillation or dry distillation of substances. It consists of a sphere, spherical vessel with a long downward-pointing neck. The liquid to be distilled is placed in the vessel and heat ...
s and receivers found at
Taxila
Taxila or Takshashila () is a city in the Pothohar region of Punjab, Pakistan. Located in the Taxila Tehsil of Rawalpindi District, it lies approximately northwest of the Islamabad–Rawalpindi metropolitan area and is just south of the ...
,
Shaikhan Dheri, and
Charsadda
Chārsadda (; ; ; ) is a town and headquarters of Charsadda District, in the Khyber Pakhtunkhwa province of Pakistan.[Pakistan
Pakistan, officially the Islamic Republic of Pakistan, is a country in South Asia. It is the List of countries and dependencies by population, fifth-most populous country, with a population of over 241.5 million, having the Islam by country# ...](_blank)
and
Rang Mahal in
India
India, officially the Republic of India, is a country in South Asia. It is the List of countries and dependencies by area, seventh-largest country by area; the List of countries by population (United Nations), most populous country since ...
dating to the early centuries of the
Common Era
Common Era (CE) and Before the Common Era (BCE) are year notations for the Gregorian calendar (and its predecessor, the Julian calendar), the world's most widely used calendar era. Common Era and Before the Common Era are alternatives to the ...
.
Frank Raymond Allchin says these terracotta distill tubes were "made to imitate bamboo". These "
Gandhara
Gandhara () was an ancient Indo-Aryan people, Indo-Aryan civilization in present-day northwest Pakistan and northeast Afghanistan. The core of the region of Gandhara was the Peshawar valley, Peshawar (Pushkalawati) and Swat valleys extending ...
stills" were only capable of producing very weak
liquor
Liquor ( , sometimes hard liquor), spirits, distilled spirits, or spiritous liquor are alcoholic drinks produced by the distillation of grains, fruits, vegetables, or sugar that have already gone through ethanol fermentation, alcoholic ferm ...
, as there was no efficient means of collecting the vapors at low heat.
[ Habib, Irfan (2011)]
''Economic History of Medieval India, 1200–1500''
Pearson Education
Pearson Education, known since 2011 as simply Pearson, is the educational publishing and services subsidiary of the international corporation Pearson plc. The subsidiary was formed in 1998, when Pearson plc acquired Simon & Schuster's educatio ...
. p. 55.
Distillation in China may have begun at the earliest during the
Eastern Han
The Han dynasty was an Dynasties of China, imperial dynasty of China (202 BC9 AD, 25–220 AD) established by Liu Bang and ruled by the House of Liu. The dynasty was preceded by the short-lived Qin dynasty (221–206 BC ...
dynasty (1st–2nd century CE).
Islamic Golden Age
Medieval
Muslim chemists such as
Jābir ibn Ḥayyān (Latin: Geber, ninth century) and
Abū Bakr al-Rāzī (Latin: Rhazes, ) experimented extensively with the distillation of various substances. The
fractional distillation
Fractional distillation is the separation of a mixture into its component parts, or fractions. Chemical compounds are separated by heating them to a temperature at which one or more fractions of the mixture will vaporize. It uses distillation ...
of organic substances plays an important role in the works attributed to Jābir, such as in the
('The Book of Seventy'), translated into Latin by
Gerard of Cremona
Gerard of Cremona (Latin: ''Gerardus Cremonensis''; c. 1114 – 1187) was an Italians, Italian translator of scientific books from Arabic into Latin. He worked in Toledo, Spain, Toledo, Kingdom of Castile and obtained the Arabic books in the libr ...
() under the title . The Jabirian experiments with fractional distillation of animal and vegetable substances, and to a lesser degree also of mineral substances, is the main topic of the , an originally Arabic work falsely attributed to
Avicenna
Ibn Sina ( – 22 June 1037), commonly known in the West as Avicenna ( ), was a preeminent philosopher and physician of the Muslim world, flourishing during the Islamic Golden Age, serving in the courts of various Iranian peoples, Iranian ...
that was translated into Latin and would go on to form the most important alchemical source for
Roger Bacon
Roger Bacon (; or ', also '' Rogerus''; ), also known by the Scholastic accolades, scholastic accolade ''Doctor Mirabilis'', was a medieval English polymath, philosopher, scientist, theologian and Franciscans, Franciscan friar who placed co ...
().
The distillation of
wine
Wine is an alcoholic drink made from Fermentation in winemaking, fermented fruit. Yeast in winemaking, Yeast consumes the sugar in the fruit and converts it to ethanol and carbon dioxide, releasing heat in the process. Wine is most often made f ...
is attested in Arabic works attributed to
al-Kindī () and to
al-Fārābī (), and in the 28th book of
al-Zahrāwī's (Latin: Abulcasis, 936–1013) ' (later translated into Latin as '). In the twelfth century, recipes for the production of ' ("burning water", i.e., ethanol) by distilling wine with
salt
In common usage, salt is a mineral composed primarily of sodium chloride (NaCl). When used in food, especially in granulated form, it is more formally called table salt. In the form of a natural crystalline mineral, salt is also known as r ...
started to appear in a number of Latin works, and by the end of the thirteenth century it had become a widely known substance among Western European chemists. The works of
Taddeo Alderotti
Taddeo Alderotti (Latin: Thaddaeus Alderottus, French : Thaddée de Florence), born in Florence between 1206 and 1215, died in 1295, was an Italian doctor and professor of medicine at the University of Bologna, who made important contributions ...
(1223–1296) describe a method for concentrating alcohol involving repeated distillation through a water-cooled still, by which an alcohol purity of 90% could be obtained.
Medieval China
The distillation of beverages began in the
Southern Song
The Song dynasty ( ) was an imperial dynasty of China that ruled from 960 to 1279. The dynasty was founded by Emperor Taizu of Song, who usurped the throne of the Later Zhou dynasty and went on to conquer the rest of the Ten Kingdoms, ending ...
(10th–13th century) and
Jin (12th–13th century) dynasties, according to archaeological evidence.
A still was found in an archaeological site in Qinglong,
Hebei
Hebei is a Provinces of China, province in North China. It is China's List of Chinese administrative divisions by population, sixth-most populous province, with a population of over 75 million people. Shijiazhuang is the capital city. It bor ...
province, China, dating back to the 12th century. Distilled beverages were common during the
Yuan dynasty
The Yuan dynasty ( ; zh, c=元朝, p=Yuáncháo), officially the Great Yuan (; Mongolian language, Mongolian: , , literally 'Great Yuan State'), was a Mongol-led imperial dynasty of China and a successor state to the Mongol Empire after Div ...
(13th–14th century).
Modern era
In 1500, German alchemist
Hieronymus Brunschwig published ' (''The Book of the Art of Distillation out of Simple Ingredients''), the first book solely dedicated to the subject of distillation, followed in 1512 by a much expanded version. Right after that, in 1518, the oldest surviving distillery in Europe,
The Green Tree Distillery, was founded.
In 1651,
John French published ''The Art of Distillation'', the first major English compendium on the practice, but it has been claimed that much of it derives from Brunschwig's work. This includes diagrams with people in them showing the industrial rather than bench scale of the operation.

]




As
alchemy
Alchemy (from the Arabic word , ) is an ancient branch of natural philosophy, a philosophical and protoscientific tradition that was historically practised in China, India, the Muslim world, and Europe. In its Western form, alchemy is first ...
evolved into the science of
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
, vessels called
retort
In a chemistry laboratory, a retort is a device used for distillation or dry distillation of substances. It consists of a sphere, spherical vessel with a long downward-pointing neck. The liquid to be distilled is placed in the vessel and heat ...
s became used for distillations. Both
alembic
An alembic (from , originating from , 'cup, beaker') is an alchemical still consisting of two vessels connected by a tube, used for distillation of liquids.
Description
The complete distilling apparatus consists of three parts:
* the "" ...
s and retorts are forms of
glassware
upTypical drinkware.
This list of glassware includes drinking vessels (drinkware), tableware used to set a table for eating a meal and generally glass items such as vases, and glasses used in the catering industry. It does not include laboratory ...
with long necks pointing to the side at a downward angle to act as air-cooled
condensers __NOTOC__
Condenser may refer to:
Heat transfer
* Condenser (heat transfer), a device or unit used to condense vapor into liquid. Specific types include:
** Heat exchanger#HVAC and refrigeration air coils, HVAC air coils
** Condenser (laboratory), ...
to
condense
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor ...
the distillate and let it drip downward for collection. Later, copper alembics were invented. Riveted joints were often kept tight by using various mixtures, for instance a dough made of rye flour. These alembics often featured a cooling system around the beak, using cold water, for instance, which made the condensation of alcohol more efficient. These were called
pot still
A pot still is a type of distillation apparatus or still used to distill liquors such as whisky or brandy. In modern (post-1850s) practice, they are not used to produce rectified spirit, because they do not separate congeners from ethanol as ...
s. Today, the retorts and pot stills have been largely supplanted by more efficient distillation methods in most industrial processes. However, the pot still is still widely used for the elaboration of some fine alcohols, such as
cognac
Cognac ( , also , ) is a variety of brandy named after the Communes of France, commune of Cognac, France. It is produced in the surrounding wine-growing region in the Departments of France, departments of Charente and Charente-Maritime.
Cogn ...
,
Scotch whisky,
Irish whiskey
Irish whiskey ( or ''uisce beatha'') is whiskey made on the island of Ireland. The word 'whiskey' (or whisky) comes from the Irish , meaning ''water of life''. Irish whiskey was once the most popular spirit in the world, though a long period of ...
,
tequila
Tequila (; ) is a liquor, distilled beverage made from the blue agave plant, primarily in the area surrounding the city of Tequila, Jalisco, Tequila northwest of Guadalajara, Jalisco, Guadalajara, and in the Jaliscan Highlands (''Los Altos (Jal ...
,
rum
Rum is a liquor made by fermenting and then distilling sugarcane molasses or sugarcane juice. The distillate, a clear liquid, is often aged in barrels of oak. Rum originated in the Caribbean in the 17th century, but today it is produced i ...
,
cachaça
''Cachaça'' () is a Liquor, distilled spirit made from fermented sugarcane juice. Also known as ''pinga'', ''caninha'', and other names, it is the most popular spirit in Brazil.Cavalcante, Messias Soares. Todos os nomes da cachaça. São Pau ...
, and some
vodka
Vodka ( ; is a clear distilled beverage, distilled alcoholic beverage. Its varieties originated in Poland and Russia. Vodka is composed mainly of water and ethanol but sometimes with traces of impurities and flavourings. Traditionally, it is ...
s. Pot stills made of various materials (wood, clay, stainless steel) are also used by
bootleggers in various countries. Small pot stills are also sold for use in the domestic production of flower water or
essential oils
An essential oil is a concentrated hydrophobic liquid containing volatile (easily evaporated at normal temperatures) chemical compounds from plants. Essential oils are also known as volatile oils, ethereal oils, aetheroleum, or simply as the o ...
.
Early forms of distillation involved batch processes using one vaporization and one condensation. Purity was improved by further distillation of the condensate. Greater volumes were processed by simply repeating the distillation. Chemists reportedly carried out as many as 500 to 600 distillations in order to obtain a pure compound.
[Othmer, D. F. (1982) "Distillation – Some Steps in its Development", in W. F. Furter (ed) ''A Century of Chemical Engineering''. ]
In the early 19th century, the basics of modern techniques, including pre-heating and
reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations. It is also used in chemistry to supply energy to Chemical ...
, were developed.
[ In 1822, Anthony Perrier developed one of the first continuous stills, and then, in 1826, Robert Stein improved that design to make his patent still. In 1830, ]Aeneas Coffey
Aeneas Coffey (1780–1852) was an Irish inventor and distiller.
Biography
Coffey was born in 1780. While his birthplace is disputed, some sources indicate he was born in Ireland, likely in County Dublin or County Wicklow, while others su ...
got a patent for improving the design even further. Coffey's continuous still may be regarded as the archetype
The concept of an archetype ( ) appears in areas relating to behavior, historical psychology, philosophy and literary analysis.
An archetype can be any of the following:
# a statement, pattern of behavior, prototype, "first" form, or a main mo ...
of modern petrochemical units. The French engineer Armand Savalle developed his steam regulator around 1846.ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
distillation, and the same and subsequent years saw developments in this theme for oils and spirits.
With the emergence of chemical engineering
Chemical engineering is an engineering field which deals with the study of the operation and design of chemical plants as well as methods of improving production. Chemical engineers develop economical commercial processes to convert raw materials ...
as a discipline at the end of the 19th century, scientific rather than empirical methods could be applied. The developing petroleum
Petroleum, also known as crude oil or simply oil, is a naturally occurring, yellowish-black liquid chemical mixture found in geological formations, consisting mainly of hydrocarbons. The term ''petroleum'' refers both to naturally occurring un ...
industry in the early 20th century provided the impetus for the development of accurate design methods, such as the McCabe–Thiele method
The McCabe–Thiele method is a technique that is commonly employed in the field of chemical engineering to model the separation of two substances by a distillation column. It uses the fact that the composition at each theoretical tray is comple ...
by Ernest Thiele and the Fenske equation. The first industrial plant in the United States to use distillation as a means of ocean desalination opened in Freeport, Texas
Freeport is a city in Brazoria County, Texas, United States, located on the Gulf of Mexico, founded in 1912. According to the 2020 United States census, 2020 census, the city population was 10,696, down from 12,049 in 2010, where Hispanic (U.S. ...
in 1961 with the hope of bringing water security
The aim of water security is to maximize the benefits of water for humans and ecosystems. The second aim is to limit the risks of destructive impacts of water to an acceptable level. These risks include too much water (flood), too little water (d ...
to the region.computer simulation
Computer simulation is the running of a mathematical model on a computer, the model being designed to represent the behaviour of, or the outcome of, a real-world or physical system. The reliability of some mathematical models can be determin ...
s of distillation columns.
Applications
The application of distillation can roughly be divided into four groups: laboratory scale, industrial distillation, distillation of herbs for perfumery and medicinals (herbal distillate
Herbal distillates, also known as floral waters, hydrosols, hydrolates, herbal waters, and essential waters, are aqueous products of hydrodistillation. They are colloidal suspensions of essential oils as well as water-soluble components obtain ...
), and food processing
Food processing is the transformation of agricultural products into food, or of one form of food into other forms. Food processing takes many forms, from grinding grain into raw flour, home cooking, and complex industrial methods used in the mak ...
. The latter two are distinctively different from the former two in that distillation is not used as a true purification method but more to transfer all volatiles
Volatility or volatile may refer to:
Chemistry
* Volatility (chemistry), a measuring tendency of a substance or liquid to vaporize easily
** Volatile organic compounds, organic or carbon compounds that can evaporate at normal temperature and pre ...
from the source materials to the distillate in the processing of beverages and herbs.
The main difference between laboratory scale distillation and industrial distillation are that laboratory scale distillation is often performed on a batch basis, whereas industrial distillation often occurs continuously. In batch distillation, the composition of the source material, the vapors of the distilling compounds, and the distillate change during the distillation. In batch distillation, a still is charged (supplied) with a batch of feed mixture, which is then separated into its component fractions, which are collected sequentially from most volatile to less volatile, with the bottoms – remaining least or non-volatile fraction – removed at the end. The still can then be recharged and the process repeated.
In continuous distillation
Continuous distillation, a form of distillation, is an ongoing separation in which a mixture is continuously (without interruption) fed into the process and separated fractions are removed continuously as output streams. Distillation is the se ...
, the source materials, vapors, and distillate are kept at a constant composition by carefully replenishing the source material and removing fractions from both vapor and liquid in the system. This results in a more detailed control of the separation process.
Idealized model
The boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
of a liquid is the temperature at which the vapor pressure
Vapor pressure or equilibrium vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. The equilibrium vapor pressure is an indicat ...
of the liquid equals the pressure around the liquid, enabling bubbles to form without being crushed. A special case is the normal boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
, where the vapor pressure of the liquid equals the ambient atmospheric pressure
Atmospheric pressure, also known as air pressure or barometric pressure (after the barometer), is the pressure within the atmosphere of Earth. The standard atmosphere (symbol: atm) is a unit of pressure defined as , which is equivalent to 1,013. ...
.
It is a misconception that in a liquid mixture at a given pressure, each component boils at the boiling point corresponding to the given pressure, allowing the vapors of each component to collect separately and purely. However, this does not occur, even in an idealized system. Idealized models of distillation are essentially governed by Raoult's law
Raoult's law ( law) is a relation of physical chemistry, with implications in thermodynamics. Proposed by French chemist François-Marie Raoult in 1887, it states that the partial pressure of each component of an ideal mixture of ''liquids'' is ...
and Dalton's law
Dalton's law (also called Dalton's law of partial pressures) states that in a mixture of non-reacting gases, the total pressure exerted is equal to the sum of the partial pressures of the individual gases. This empirical law was observed by John ...
and assume that vapor–liquid equilibria are attained.
Raoult's law states that the vapor pressure of a solution is dependent on 1) the vapor pressure of each chemical component in the solution and 2) the fraction of solution each component makes up, a.k.a. the mole fraction
In chemistry, the mole fraction or molar fraction, also called mole proportion or molar proportion, is a quantity defined as the ratio between the amount of a constituent substance, ''ni'' (expressed in unit of moles, symbol mol), and the to ...
. This law applies to ideal solution
An ideal solution or ideal mixture is a solution that exhibits thermodynamic properties analogous to those of a mixture of ideal gases. The enthalpy of mixing is zero as is the volume change on mixing. The vapor pressures of all components obey R ...
s, or solutions that have different components but whose molecular interactions are the same as or very similar to pure solutions.
Dalton's law states that the total pressure is the sum of the partial pressures of each individual component in the mixture. When a multi-component liquid is heated, the vapor pressure of each component will rise, thus causing the total vapor pressure to rise. When the total vapor pressure reaches the pressure surrounding the liquid, boiling
Boiling or ebullition is the rapid phase transition from liquid to gas or vapor, vapour; the reverse of boiling is condensation. Boiling occurs when a liquid is heated to its boiling point, so that the vapour pressure of the liquid is equal to ...
occurs and liquid turns to gas throughout the bulk of the liquid. A mixture with a given composition has one boiling point at a given pressure when the components are mutually soluble. A mixture of constant composition does not have multiple boiling points.
An implication of one boiling point is that lighter components never cleanly "boil first". At boiling point, all volatile components boil, but for a component, its percentage in the vapor is the same as its percentage of the total vapor pressure. Lighter components have a higher partial pressure and, thus, are concentrated in the vapor, but heavier volatile components also have a (smaller) partial pressure and necessarily vaporize also, albeit at a lower concentration in the vapor. Indeed, batch distillation and fractionation succeed by varying the composition of the mixture. In batch distillation, the batch vaporizes, which changes its composition; in fractionation, liquid higher in the fractionation column contains more lights and boils at lower temperatures. Therefore, starting from a given mixture, it appears to have a boiling range instead of a boiling point, although this is because its composition changes: each intermediate mixture has its own, singular boiling point.
The idealized model is accurate in the case of chemically similar liquids, such as benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
and toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon with the chemical formula , often abbreviated as , where Ph stands for the phenyl group. It is a colorless, water
Water is an inorganic compound with the c ...
. In other cases, severe deviations from Raoult's law and Dalton's law are observed, most famously in the mixture of ethanol and water. These compounds, when heated together, form an azeotrope
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This happens beca ...
, which is when the vapor phase and liquid phase contain the same composition. Although there are computational methods that can be used to estimate the behavior of a mixture of arbitrary components, the only way to obtain accurate vapor–liquid equilibrium
In thermodynamics and chemical engineering, the vapor–liquid equilibrium (VLE) describes the distribution of a chemical species between the vapor phase and a liquid phase.
The Vapor quality, concentration of a vapor in contact with its liquid, ...
data is by measurement.
It is not possible to completely purify a mixture of components by distillation, as this would require each component in the mixture to have a zero partial pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal g ...
. If ultra-pure products are the goal, then further chemical separation must be applied. When a binary mixture is vaporized and the other component, e.g., a salt, has zero partial pressure for practical purposes, the process is simpler.
Batch or differential distillation
 Heating an ideal mixture of two volatile substances, A and B, with A having the higher volatility, or lower boiling point, in a batch distillation setup (such as in an apparatus depicted in the opening figure) until the mixture is boiling results in a vapor above the liquid that contains a mixture of A and B. The ratio between A and B in the vapor will be different from the ratio in the liquid. The ratio in the liquid will be determined by how the original mixture was prepared, while the ratio in the vapor will be enriched in the more volatile compound, A (due to Raoult's Law, see above). The vapor goes through the condenser and is removed from the system. This, in turn, means that the ratio of compounds in the remaining liquid is now different from the initial ratio (i.e., more enriched in B than in the starting liquid).
The result is that the ratio in the liquid mixture is changing, becoming richer in component B. This causes the boiling point of the mixture to rise, which results in a rise in the temperature in the vapor, which results in a changing ratio of A : B in the gas phase (as distillation continues, there is an increasing proportion of B in the gas phase). This results in a slowly changing ratio of A : B in the distillate.
If the difference in vapour pressure between the two components A and B is large – generally expressed as the difference in boiling points – the mixture in the beginning of the distillation is highly enriched in component A, and when component A has distilled off, the boiling liquid is enriched in component B.
Heating an ideal mixture of two volatile substances, A and B, with A having the higher volatility, or lower boiling point, in a batch distillation setup (such as in an apparatus depicted in the opening figure) until the mixture is boiling results in a vapor above the liquid that contains a mixture of A and B. The ratio between A and B in the vapor will be different from the ratio in the liquid. The ratio in the liquid will be determined by how the original mixture was prepared, while the ratio in the vapor will be enriched in the more volatile compound, A (due to Raoult's Law, see above). The vapor goes through the condenser and is removed from the system. This, in turn, means that the ratio of compounds in the remaining liquid is now different from the initial ratio (i.e., more enriched in B than in the starting liquid).
The result is that the ratio in the liquid mixture is changing, becoming richer in component B. This causes the boiling point of the mixture to rise, which results in a rise in the temperature in the vapor, which results in a changing ratio of A : B in the gas phase (as distillation continues, there is an increasing proportion of B in the gas phase). This results in a slowly changing ratio of A : B in the distillate.
If the difference in vapour pressure between the two components A and B is large – generally expressed as the difference in boiling points – the mixture in the beginning of the distillation is highly enriched in component A, and when component A has distilled off, the boiling liquid is enriched in component B.
Continuous distillation
Continuous distillation is an ongoing distillation in which a liquid mixture is continuously (without interruption) fed into the process and separated fractions are removed continuously as output streams occur over time during the operation. Continuous distillation produces a minimum of two output fractions, including at least one volatile distillate fraction, which has boiled and been separately captured as a vapor and then condensed to a liquid. There is always a bottoms (or residue) fraction, which is the least volatile residue that has not been separately captured as a condensed vapor.
Continuous distillation differs from batch distillation in the respect that concentrations should not change over time. Continuous distillation can be run at a steady state
In systems theory, a system or a process is in a steady state if the variables (called state variables) which define the behavior of the system or the process are unchanging in time. In continuous time, this means that for those properties ''p' ...
for an arbitrary amount of time. For any source material of specific composition, the main variables that affect the purity of products in continuous distillation are the reflux ratio and the number of theoretical equilibrium stages, in practice determined by the number of trays or the height of packing. Reflux is a flow from the condenser back to the column, which generates a recycle that allows a better separation with a given number of trays. Equilibrium stages are ideal steps where compositions achieve vapor–liquid equilibrium, repeating the separation process and allowing better separation given a reflux ratio. A column with a high reflux ratio may have fewer stages, but it refluxes a large amount of liquid, giving a wide column with a large holdup. Conversely, a column with a low reflux ratio must have a large number of stages, thus requiring a taller column.
General improvements
Both batch and continuous distillations can be improved by making use of a fractionating column
A fractionating column or fractional column is equipment used in the distillation of liquid mixtures to separate the mixture into its component parts, or fractions, based on their differences in volatility. Fractionating columns are used in small ...
on top of the distillation flask. The column improves separation by providing a larger surface area for the vapor and condensate to come into contact. This helps it remain at equilibrium for as long as possible. The column can even consist of small subsystems ('trays' or 'dishes') which all contain an enriched, boiling liquid mixture, all with their own vapor–liquid equilibrium.
There are differences between laboratory-scale and industrial-scale fractionating columns, but the principles are the same. Examples of laboratory-scale fractionating columns (in increasing efficiency) include:
* Air condenser
* Vigreux column (usually laboratory scale only)
* Packed column (packed with glass beads, metal pieces, or other chemically inert material)
* Spinning band distillation system.
Laboratory procedures
Laboratory scale distillations are almost exclusively run as batch distillations. The device used in distillation, sometimes referred to as a ''still
A still is an apparatus used to distillation, distill liquid mixtures by heating to selectively Boiling, boil and then cooling to Condensation, condense the vapor. A still uses the same concepts as a basic Distillation#Laboratory_procedures, ...
'', consists at a minimum of a reboiler or ''pot'' in which the source material is heated, a condenser in which the heated vapor
In physics, a vapor (American English) or vapour (Commonwealth English; American and British English spelling differences#-our, -or, see spelling differences) is a substance in the gas phase at a temperature lower than its critical temperature,R ...
is cooled back to the liquid state
State most commonly refers to:
* State (polity), a centralized political organization that regulates law and society within a territory
**Sovereign state, a sovereign polity in international law, commonly referred to as a country
**Nation state, a ...
, and a receiver in which the concentrated or purified liquid, called the distillate, is collected. Several laboratory scale techniques for distillation exist (see also distillation types).
A completely sealed distillation apparatus could experience extreme and rapidly varying internal pressure, which could cause it to burst open at the joints. Therefore, some path is usually left open (for instance, at the receiving flask) to allow the internal pressure to equalize with atmospheric pressure. Alternatively, a vacuum pump
A vacuum pump is a type of pump device that draws gas particles from a sealed volume in order to leave behind a partial vacuum. The first vacuum pump was invented in 1650 by Otto von Guericke, and was preceded by the suction pump, which dates to ...
may be used to keep the apparatus at a lower than atmospheric pressure. If the substances involved are air- or moisture-sensitive, the connection to the atmosphere can be made through one or more drying tubes packed with materials that scavenge the undesired air components, or through bubblers that provide a movable liquid barrier. Finally, the entry of undesired air components can be prevented by pumping a low but steady flow of suitable inert gas, like nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
, into the apparatus.
Simple distillation
 In simple distillation, the vapor is immediately channeled into a condenser. Consequently, the distillate is not pure but rather its composition is identical to the composition of the vapors at the given temperature and pressure. That concentration follows
In simple distillation, the vapor is immediately channeled into a condenser. Consequently, the distillate is not pure but rather its composition is identical to the composition of the vapors at the given temperature and pressure. That concentration follows Raoult's law
Raoult's law ( law) is a relation of physical chemistry, with implications in thermodynamics. Proposed by French chemist François-Marie Raoult in 1887, it states that the partial pressure of each component of an ideal mixture of ''liquids'' is ...
.
As a result, simple distillation is effective only when the liquid boiling points differ greatly (rule of thumb is 25 °C) or when separating liquids from non-volatile solids or oils. For these cases, the vapor pressures of the components are usually different enough that the distillate may be sufficiently pure for its intended purpose.
A cutaway schematic of a simple distillation operation is shown at right. The starting liquid 15 in the boiling flask 2 is heated by a combined hotplate
A hot plate or hotplate is a heated flat surface on a stove or electric cooker on which food may be cooked, either built into an electric cooker or kitchen stove, or portable, plugged into an electric outlet.
Hot plates can also be used as a h ...
and magnetic stirrer
A magnetic stirrer or magnetic mixer is a laboratory device that employs a rotating magnetic field to cause a stir bar (or ''flea'') immersed in a liquid to spin very quickly, thus stirring it. The rotating field may be created either by a rota ...
13 via a silicone oil
A silicone oil is any liquid polymerized siloxane with organic side chains. The most important member is polydimethylsiloxane. These polymers are of commercial interest because of their relatively high thermal stability and their lubricating prop ...
bath (orange, 14). The vapor flows through a short Vigreux column 3, then through a Liebig condenser
The Liebig condenser (, ) or straight condenser is a piece of laboratory equipment, specifically a condenser (laboratory), condenser consisting of a straight glass tube surrounded by a water jacket.
In typical laboratory operation, such as distil ...
5, is cooled by water (blue) that circulates through ports 6 and 7. The condensed liquid drips into the receiving flask 8, sitting in a cooling bath (blue, 16). The adapter 10 has a connection 9 that may be fitted to a vacuum pump. The components are connected by ground glass joint
Ground glass joints are used in laboratories to quickly and easily fit leak-tight apparatus together from interchangeable commonly available parts. For example, a round bottom flask, Liebig condenser, and oil bubbler with ground glass joints may ...
s.
Fractional distillation
For many cases, the boiling points of the components in the mixture will be sufficiently close that Raoult's law
Raoult's law ( law) is a relation of physical chemistry, with implications in thermodynamics. Proposed by French chemist François-Marie Raoult in 1887, it states that the partial pressure of each component of an ideal mixture of ''liquids'' is ...
must be taken into consideration. Therefore, fractional distillation must be used to separate the components by repeated vaporization-condensation cycles within a packed fractionating column. This separation, by successive distillations, is also referred to as rectification.[
As the solution to be purified is heated, its vapors rise to the ]fractionating column
A fractionating column or fractional column is equipment used in the distillation of liquid mixtures to separate the mixture into its component parts, or fractions, based on their differences in volatility. Fractionating columns are used in small ...
. As it rises, it cools, condensing on the condenser walls and the surfaces of the packing material. Here, the condensate continues to be heated by the rising hot vapors; it vaporizes once more. However, the composition of the fresh vapors is determined once again by Raoult's law. Each vaporization-condensation cycle (called a ''theoretical plate
A theoretical plate in many separation processes is a hypothetical zone or stage in which two phases, such as the liquid and vapor phases of a substance, establish an equilibrium with each other. Such equilibrium stages may also be referred to as ...
'') will yield a purer solution of the more volatile component. In reality, each cycle at a given temperature does not occur at exactly the same position in the fractionating column; ''theoretical plate'' is thus a concept rather than an accurate description.
More theoretical plates lead to better separations. A spinning band distillation system uses a spinning band of PTFE
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene, and has numerous applications because it is chemically inert. The commonly known brand name of PTFE-based composition is Teflon by Chemours, a spin-off fro ...
or metal to force the rising vapors into close contact with the descending condensate, increasing the number of theoretical plates.
Steam distillation
Like vacuum distillation
Vacuum distillation or distillation under reduced pressure is a type of distillation performed under reduced pressure, which allows the purification of compounds not readily distilled at ambient pressures or simply to save time or energy. This te ...
, steam distillation is a method for distilling compounds which are heat-sensitive.heating element
A heating element is a device used for conversion of electric energy into heat, consisting of a heating resistor and accessories. Heat is generated by the passage of electric current through a resistor through a process known as Joule heating. He ...
and allows a high rate of heat transfer without heating at a very high temperature. This process involves bubbling steam through a heated mixture of the raw material. By Raoult's law, some of the target compound will vaporize (in accordance with its partial pressure). The vapor mixture is cooled and condensed, usually yielding a layer of oil and a layer of water.
Steam distillation of various aroma
An odor (American English) or odour (Commonwealth English; see spelling differences) is a smell or a scent caused by one or more volatilized chemical compounds generally found in low concentrations that humans and many animals can perceive v ...
tic herbs and flowers can result in two products: an essential oil
An essential oil is a concentrated hydrophobic liquid containing volatile (easily evaporated at normal temperatures) chemical compounds from plants. Essential oils are also known as volatile oils, ethereal oils, aetheroleum, or simply as the ...
as well as a watery herbal distillate
Herbal distillates, also known as floral waters, hydrosols, hydrolates, herbal waters, and essential waters, are aqueous products of hydrodistillation. They are colloidal suspensions of essential oils as well as water-soluble components obtain ...
. The essential oils
An essential oil is a concentrated hydrophobic liquid containing volatile (easily evaporated at normal temperatures) chemical compounds from plants. Essential oils are also known as volatile oils, ethereal oils, aetheroleum, or simply as the o ...
are often used in perfumery and aromatherapy
Aromatherapy is a practice based on the use of aromatic materials, including essential oils and other aroma compounds, with claims for improving psychological well-being. It is used as a complementary therapy or as a form of alternative medic ...
while the watery distillates have many applications in aromatherapy
Aromatherapy is a practice based on the use of aromatic materials, including essential oils and other aroma compounds, with claims for improving psychological well-being. It is used as a complementary therapy or as a form of alternative medic ...
, food processing
Food processing is the transformation of agricultural products into food, or of one form of food into other forms. Food processing takes many forms, from grinding grain into raw flour, home cooking, and complex industrial methods used in the mak ...
and skin care
Skin care or skincare is the practice of maintaining and improving the health and appearance of the skin. It includes washing, moisturizing, protecting from the sun, and treating skin problems like acne and dryness. Skin care can help prevent i ...
.

Vacuum distillation
Some compounds have very high boiling points. To boil such compounds, it is often better to lower the pressure at which such compounds are boiled instead of increasing the temperature. Once the pressure is lowered to the vapor pressure of the compound (at the given temperature), boiling and the rest of the distillation process can commence. This technique is referred to as vacuum distillation and it is commonly found in the laboratory in the form of the rotary evaporator.
This technique is also very useful for compounds which boil beyond their decomposition temperature
Thermal decomposition, or thermolysis, is a chemical decomposition of a substance caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic ...
at atmospheric pressure and which would therefore be decomposed by any attempt to boil them under atmospheric pressure.
Molecular distillation
Molecular distillation Molecular distillation is a type of short-path vacuum distillation, characterized by an extremely low vacuum pressure, 0.01 torr or below, which is performed using a molecular still. It is a process of separation, purification and concentration of ...
is vacuum distillation below the pressure of 0.01 torr
The torr (symbol: Torr) is a Pressure#Units, unit of pressure based on an absolute scale, defined as exactly of a standard atmosphere (unit), atmosphere (101325 Pa). Thus one torr is exactly (≈ ).
Historically, one torr was intended to be ...
. 0.01 torr is one order of magnitude above high vacuum, where fluids are in the free molecular flow
Free molecular flow describes the fluid dynamics of gas where the mean free path of the molecules is larger than the size of the chamber or of the object under test. For tubes/objects of the size of several cm, this means pressures well below 10− ...
regime, i.e., the mean free path
In physics, mean free path is the average distance over which a moving particle (such as an atom, a molecule, or a photon) travels before substantially changing its direction or energy (or, in a specific context, other properties), typically as a ...
of molecules is comparable to the size of the equipment. The gaseous phase no longer exerts significant pressure on the substance to be evaporated, and consequently, rate of evaporation no longer depends on pressure. That is, because the continuum assumptions of fluid dynamics no longer apply, mass transport is governed by molecular dynamics rather than fluid dynamics. Thus, a short path between the hot surface and the cold surface is necessary, typically by suspending a hot plate covered with a film of feed next to a cold plate with a line of sight in between. Molecular distillation is used industrially for purification of oils.
Short path distillation
Short path distillation is a distillation technique that involves the distillate travelling a short distance, often only a few centimeters, and is normally done at reduced pressure.
Air-sensitive vacuum distillation
Some compounds have high boiling points as well as being air sensitive Air sensitivity is a term used, particularly in chemistry, to denote the reactivity of chemical compounds with some constituent of air. Most often, reactions occur with atmospheric oxygen (O2) or water vapor (H2O), although reactions with the other ...
. A simple vacuum distillation system as exemplified above can be used, whereby the vacuum is replaced with an inert gas after the distillation is complete. However, this is a less satisfactory system if one desires to collect fractions under a reduced pressure. To do this a "cow" or "pig" adaptor can be added to the end of the condenser, or for better results or for very air sensitive compounds a Perkin triangle apparatus can be used.
The Perkin triangle has means via a series of glass or Teflon
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene, and has numerous applications because it is chemically inert. The commonly known brand name of PTFE-based composition is Teflon by Chemours, a spin-off from ...
taps to allows fractions to be isolated from the rest of the still
A still is an apparatus used to distillation, distill liquid mixtures by heating to selectively Boiling, boil and then cooling to Condensation, condense the vapor. A still uses the same concepts as a basic Distillation#Laboratory_procedures, ...
, without the main body of the distillation being removed from either the vacuum or heat source, and thus can remain in a state of reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations. It is also used in chemistry to supply energy to Chemical ...
. To do this, the sample is first isolated from the vacuum by means of the taps, the vacuum over the sample is then replaced with an inert gas (such as nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
or argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
) and can then be stoppered and removed. A fresh collection vessel can then be added to the system, evacuated and linked back into the distillation system via the taps to collect a second fraction, and so on, until all fractions have been collected.
Zone distillation
Zone distillation is a distillation process in a long container with partial melting of refined matter in moving liquid zone and condensation of vapor in the solid phase at condensate pulling in cold area. The process is worked in theory. When zone heater is moving from the top to the bottom of the container then solid condensate with irregular impurity distribution is forming. Then most pure part of the condensate may be extracted as product. The process may be iterated many times by moving (without turnover) the received condensate to the bottom part of the container on the place of refined matter. The irregular impurity distribution in the condensate (that is efficiency of purification) increases with the number of iterations.
Zone distillation is the distillation analog of zone recrystallization. Impurity distribution in the condensate is described by known equations of zone recrystallization – with the replacement of the distribution co-efficient k of crystallization - for the separation factor α of distillation.
Closed-system vacuum distillation (cryovap)
Non-condensable gas can be expelled from the apparatus by the vapor of relatively volatile co-solvent, which spontaneously evaporates during initial pumping, and this can be achieved with regular oil or diaphragm pump.
Other types
* The process of reactive distillation involves using the reaction vessel as the still. In this process, the product is usually significantly lower boiling than its reactants. As the product is formed from the reactants, it is vaporized and removed from the reaction mixture. This technique is an example of a continuous vs. a batch process; advantages include less downtime to charge the reaction vessel with starting material, and less workup. Distillation "over a reactant" could be classified as a reactive distillation. It is typically used to remove volatile impurity from the distillation feed. For example, a little lime may be added to remove carbon dioxide from water followed by a second distillation with a little sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
added to remove traces of ammonia.
* Catalytic distillation is the process by which the reactants are catalyzed while being distilled to continuously separate the products from the reactants. This method is used to assist equilibrium reactions in reaching completion.
* Pervaporation Pervaporation (or pervaporative separation) is a processing method for the separation of mixtures of liquids by partial vaporization through a non-porous or porous membrane.
Theory
The term ''pervaporation'' is a portmanteau of the two steps of ...
is a method for the separation of mixtures of liquids by partial vaporization through a non-porous membrane
A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Membranes can be generally classified into synthetic membranes and biological membranes. Bi ...
.
* Extractive distillation is defined as distillation in the presence of a miscible, high boiling, relatively non-volatile component, the solvent, that forms no azeotrope
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This happens beca ...
with the other components in the mixture.
* Flash evaporation (or partial evaporation) is the partial vaporization
Vaporization (or vapo(u)risation) of an element or compound is a phase transition from the liquid phase to vapor. There are two types of vaporization: evaporation and boiling. Evaporation is a surface phenomenon, whereas boiling is a bulk phenome ...
that occurs when a saturated liquid stream undergoes a reduction in pressure by passing through a throttling valve
A valve is a device or natural object that regulates, directs or controls the flow of a fluid (gases, liquids, fluidized solids, or Slurry, slurries) by opening, closing, or partially obstructing various passageways. Valves are technically Pip ...
or other throttling device. This process is one of the simplest unit operations, being equivalent to a distillation with only one equilibrium stage.
* Codistillation is distillation which is performed on mixtures in which the two compounds are not miscible. In the laboratory, the Dean-Stark apparatus is used for this purpose to remove water from synthesis products. The Bleidner apparatus is another example with two refluxing solvents.
* Membrane distillation is a type of distillation in which vapors of a mixture to be separated are passed through a membrane, which selectively permeates one component of mixture. Vapor pressure difference is the driving force. It has potential applications in seawater desalination and in removal of organic and inorganic components.
The unit process of evaporation
Evaporation is a type of vaporization that occurs on the Interface (chemistry), surface of a liquid as it changes into the gas phase. A high concentration of the evaporating substance in the surrounding gas significantly slows down evapora ...
may also be called "distillation":
* In rotary evaporation a vacuum distillation apparatus is used to remove bulk solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
s from a sample. Typically the vacuum is generated by a water aspirator or a membrane pump.
* In a Kugelrohr apparatus a short path distillation apparatus is typically used (generally in combination with a (high) vacuum) to distill high boiling (> 300 °C) compounds. The apparatus consists of an oven in which the compound to be distilled is placed, a receiving portion which is outside of the oven, and a means of rotating the sample. The vacuum is normally generated by using a high vacuum pump.
Other uses:
* Dry distillation
Dry distillation is the heating of solid materials to produce gaseous products (which may condense into liquids or solids). The method may involve pyrolysis or thermolysis, or it may not (for instance, a simple mixture of ice and glass could be ...
or destructive distillation
Destructive distillation is a chemical process in which decomposition of unprocessed material is achieved by heating it to a high temperature; the term generally applies to processing of organic material in the absence of air or in the presence o ...
, despite the name, is not truly distillation, but rather a chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
known as pyrolysis
Pyrolysis is a process involving the Bond cleavage, separation of covalent bonds in organic matter by thermal decomposition within an Chemically inert, inert environment without oxygen. Etymology
The word ''pyrolysis'' is coined from the Gree ...
in which solid substances are heated in an inert or reducing atmosphere and any volatile fractions, containing high-boiling liquids and products of pyrolysis, are collected. The destructive distillation of wood
Wood is a structural tissue/material found as xylem in the stems and roots of trees and other woody plants. It is an organic materiala natural composite of cellulosic fibers that are strong in tension and embedded in a matrix of lignin t ...
to give methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
is the root of its common name – ''wood alcohol''.
* Freeze distillation
Fractional freezing is a process used in process engineering and chemistry to separate substances with different melting points. It can be done by partial melting of a solid, for example in zone refining of silicon or metals, or by partial cry ...
is an analogous method of purification using freezing
Freezing is a phase transition in which a liquid turns into a solid when its temperature is lowered below its freezing point.
For most substances, the melting and freezing points are the same temperature; however, certain substances possess dif ...
instead of evaporation. It is not truly distillation, but a recrystallization where the product is the mother liquor
The mother liquor (or spent liquor) is the Solution (chemistry), solution remaining after a component has been removed by a process such as filtration or more commonly crystallization. It is encountered in chemical processes including sugar refini ...
, and does not produce products equivalent to distillation. This process is used in the production of ice beer
Ice beer is a beer that has undergone some degree of freezing during production. These beers generally have a higher alcohol content, and lower price relative to it.
The process of "icing" beer involves lowering the temperature until ice crystals ...
and ice wine
Icewine (or ice wine; ) is a type of dessert wine produced from grapes that have been Freezing, frozen while still on the vine. The sugars and other dissolved solids do not freeze, but the water does, allowing for a more concentrated grape juice ...
to increase ethanol and sugar
Sugar is the generic name for sweet-tasting, soluble carbohydrates, many of which are used in food. Simple sugars, also called monosaccharides, include glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecul ...
content, respectively. It is also used to produce applejack. Unlike distillation, freeze distillation concentrates poisonous congeners rather than removing them; As a result, many countries prohibit such applejack as a health measure. Also, distillation by evaporation can separate these since they have different boiling points.
* Distillation by filtration: In early alchemy and chemistry, otherwise known as natural philosophy, a form of "distillation" by capillary filtration was known as a form of distillation at the time. In this, a series of cups or bowls were set upon a stepped support with a "wick" of cotton or felt-like material, which had been wetted with water or a clear liquid with each step dripping down through the wetted cloth through capillary action in succeeding steps, creating a "purification" of the liquid, leaving solid materials behind in the upper bowls and purifying the succeeding product through capillary action through the moistened cloth. This was called "distillatio" by filtration by those using the method.
Azeotropic process
Interactions between the components of the solution create properties unique to the solution, as most processes entail non-ideal mixtures, where Raoult's law
Raoult's law ( law) is a relation of physical chemistry, with implications in thermodynamics. Proposed by French chemist François-Marie Raoult in 1887, it states that the partial pressure of each component of an ideal mixture of ''liquids'' is ...
does not hold. Such interactions can result in a constant-boiling azeotrope
An azeotrope () or a constant heating point mixture is a mixture of two or more liquids whose proportions cannot be changed by simple distillation.Moore, Walter J. ''Physical Chemistry'', 3rd e Prentice-Hall 1962, pp. 140–142 This happens beca ...
which behaves as if it were a pure compound (i.e., boils at a single temperature instead of a range). At an azeotrope, the solution contains the given component in the same proportion as the vapor, so that evaporation does not change the purity, and distillation does not result in separation. For example, 95.6% ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
(by mass) in water forms an azeotrope at 78.1 °C.
If the azeotrope is not considered sufficiently pure for use, there exist some techniques to break the azeotrope to give a more pure distillate. These techniques are known as azeotropic distillation. Some techniques achieve this by "jumping" over the azeotropic composition (by adding another component to create a new azeotrope, or by varying the pressure). Others work by chemically or physically removing or sequestering the impurity. For example, to purify ethanol beyond 95%, a drying agent (or desiccant
A desiccant is a hygroscopic substance that is used to induce or sustain a state of dryness (desiccation) in its vicinity; it is the opposite of a humectant. Commonly encountered pre-packaged desiccants are solids that absorb water. Desiccant ...
, such as potassium carbonate
Potassium carbonate is the inorganic compound with the formula . It is a white salt, which is soluble in water and forms a strongly alkaline solution. It is deliquescent, often appearing as a damp or wet solid. Potassium carbonate is mainly used ...
) can be added to convert the soluble water into insoluble water of crystallization
In chemistry, water(s) of crystallization or water(s) of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. In some contexts, water of crystallization is ...
. Molecular sieve
A molecular sieve is a material with pores of uniform size comparable to that of individual molecules, linking the interior of the solid to its exterior. These materials embody the molecular sieve effect, in which molecules larger than the pore ...
s are often used for this purpose as well.
Immiscible
Miscibility () is the property of two chemical substance, substances to mix in all mixing ratio, proportions (that is, to fully dissolution (chemistry), dissolve in each other at any concentration), forming a homogeneity and heterogeneity, homoge ...
liquids, such as water and toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon with the chemical formula , often abbreviated as , where Ph stands for the phenyl group. It is a colorless, water
Water is an inorganic compound with the c ...
, easily form azeotropes. Commonly, these azeotropes are referred to as a low boiling azeotrope because the boiling point of the azeotrope is lower than the boiling point of either pure component. The temperature and composition of the azeotrope is easily predicted from the vapor pressure of the pure components, without use of Raoult's law. The azeotrope is easily broken in a distillation set-up by using a liquid–liquid separator (a decanter) to separate the two liquid layers that are condensed overhead. Only one of the two liquid layers is refluxed to the distillation set-up.
High boiling azeotropes, such as a 20 percent by weight mixture of hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
in water, also exist. As implied by the name, the boiling point of the azeotrope is greater than the boiling point of either pure component.
Breaking an azeotrope with unidirectional pressure manipulation
The boiling points of components in an azeotrope overlap to form a band. By exposing an azeotrope to a vacuum or positive pressure, it is possible to bias the boiling point of one component away from the other by exploiting the differing vapor pressure curves of each; the curves may overlap at the azeotropic point, but are unlikely to remain identical further along the pressure axis to either side of the azeotropic point. When the bias is great enough, the two boiling points no longer overlap and so the azeotropic band disappears.
This method can remove the need to add other chemicals to a distillation, but it has two potential drawbacks.
Under negative pressure, power for a vacuum source is needed and the reduced boiling points of the distillates requires that the condenser be run cooler to prevent distillate vapors being lost to the vacuum source. Increased cooling demands will often require additional energy and possibly new equipment or a change of coolant.
Alternatively, if positive pressures are required, standard glassware can not be used, energy must be used for pressurization and there is a higher chance of side reactions occurring in the distillation, such as decomposition, due to the higher temperatures required to effect boiling.
A unidirectional distillation will rely on a pressure change in one direction, either positive or negative.
Pressure-swing distillation
Pressure-swing distillation is essentially the same as the unidirectional distillation used to break azeotropic mixtures, but here both positive and negative pressures may be employed.
This improves the selectivity of the distillation and allows a chemist to optimize distillation by avoiding extremes of pressure and temperature that waste energy. This is particularly important in commercial applications.
One example of the application of pressure-swing distillation is during the industrial purification of ethyl acetate
Ethyl acetate commonly abbreviated EtOAc, ETAC or EA) is the organic compound with the formula , simplified to . This flammable, colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues, nail polish removers, ...
after its catalytic synthesis from ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the ps ...
.
Industrial process
 Large scale industrial distillation applications include both batch and continuous fractional, vacuum, azeotropic, extractive, and steam distillation. The most widely used industrial applications of continuous, steady-state fractional distillation are in
Large scale industrial distillation applications include both batch and continuous fractional, vacuum, azeotropic, extractive, and steam distillation. The most widely used industrial applications of continuous, steady-state fractional distillation are in petroleum refineries
An oil refinery or petroleum refinery is an industrial process plant where petroleum (crude oil) is transformed and refined into products such as gasoline (petrol), diesel fuel, asphalt base, fuel oils, heating oil, kerosene, liquefied petr ...
, petrochemical
Petrochemicals (sometimes abbreviated as petchems) are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable s ...
and chemical plant
A chemical plant is an industrial process plant that manufactures (or otherwise processes) chemicals, usually on a large scale. The general objective of a chemical plant is to create new material wealth via the chemical or biological transfor ...
s and natural gas processing
Nature is an inherent character or constitution, particularly of the ecosphere or the universe as a whole. In this general sense nature refers to the laws, elements and phenomena of the physical world, including life. Although humans are part ...
plants.
To control and optimize such industrial distillation, a standardized laboratory method, ASTM D86, is established. This test method extends to the atmospheric distillation of petroleum products using a laboratory batch distillation unit to quantitatively determine the boiling range characteristics of petroleum products.
Industrial distillationcrude oil
Petroleum, also known as crude oil or simply oil, is a naturally occurring, yellowish-black liquid chemical mixture found in geological formations, consisting mainly of hydrocarbons. The term ''petroleum'' refers both to naturally occurring u ...
, liquid outlets at intervals up the column allow for the withdrawal of different ''fractions'' or products having different boiling points
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envi ...
or boiling ranges. The "lightest" products (those with the lowest boiling point) exit from the top of the columns and the "heaviest" products (those with the highest boiling point) exit from the bottom of the column and are often called the bottoms.
 Industrial towers use
Industrial towers use reflux
Reflux is a technique involving the condensation of vapors and the return of this condensate to the system from which it originated. It is used in industrial and laboratory distillations. It is also used in chemistry to supply energy to Chemical ...
to achieve a more complete separation of products. Reflux refers to the portion of the condensed overhead liquid product from a distillation or fractionation tower that is returned to the upper part of the tower as shown in the schematic diagram of a typical, large-scale industrial distillation tower. Inside the tower, the downflowing reflux liquid provides cooling and condensation of the upflowing vapors thereby increasing the efficiency of the distillation tower. The more reflux that is provided for a given number of theoretical plate
A theoretical plate in many separation processes is a hypothetical zone or stage in which two phases, such as the liquid and vapor phases of a substance, establish an equilibrium with each other. Such equilibrium stages may also be referred to as ...
s, the better the tower's separation of lower boiling materials from higher boiling materials. Alternatively, the more reflux that is provided for a given desired separation, the fewer the number of theoretical plates required. Chemical engineer
A chemical engineer is a professional equipped with the knowledge of chemistry and other basic sciences who works principally in the chemical industry to convert basic raw materials into a variety of Product (chemistry), products and deals with ...
s must choose what combination of reflux rate and number of plates is both economically and physically feasible for the products purified in the distillation column.
Such industrial fractionating towers are also used in cryogenic
In physics, cryogenics is the production and behaviour of materials at very low temperatures.
The 13th International Institute of Refrigeration's (IIR) International Congress of Refrigeration (held in Washington, DC in 1971) endorsed a univers ...
air separation
An air separation plant separates Atmosphere of Earth, atmospheric air into its primary components, typically nitrogen and oxygen, and sometimes also argon and other rare inert gases.
The most common method for air separation is fractional distill ...
, producing liquid oxygen
Liquid oxygen, sometimes abbreviated as LOX or LOXygen, is a clear cyan liquid form of dioxygen . It was used as the oxidizer in the first liquid-fueled rocket invented in 1926 by Robert H. Goddard, an application which is ongoing.
Physical ...
, liquid nitrogen
Liquid nitrogen (LN2) is nitrogen in a liquid state at cryogenics, low temperature. Liquid nitrogen has a boiling point of about . It is produced industrially by fractional distillation of liquid air. It is a colorless, mobile liquid whose vis ...
, and high purity argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abu ...
. Distillation of chlorosilane
In inorganic chemistry, chlorosilanes are a group of reactive, chlorine-containing chemical compounds, related to silane () and used in many chemical processes. Each such chemical has at least one silicon-chlorine () bond. Trichlorosilane is pro ...
s also enables the production of high-purity silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
for use as a semiconductor
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping level ...
.
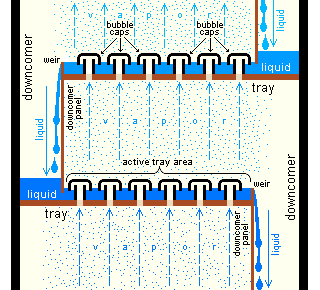 Design and operation of a distillation tower depends on the feed and desired products. Given a simple, binary component feed, analytical methods such as the
Design and operation of a distillation tower depends on the feed and desired products. Given a simple, binary component feed, analytical methods such as the McCabe–Thiele method
The McCabe–Thiele method is a technique that is commonly employed in the field of chemical engineering to model the separation of two substances by a distillation column. It uses the fact that the composition at each theoretical tray is comple ...
[ can be used. For a multi-component feed, ]simulation
A simulation is an imitative representation of a process or system that could exist in the real world. In this broad sense, simulation can often be used interchangeably with model. Sometimes a clear distinction between the two terms is made, in ...
models are used both for design and operation. Moreover, the efficiencies of the vapor–liquid contact devices (referred to as "plates" or "trays") used in distillation towers are typically lower than that of a theoretical 100% efficient equilibrium stage
A theoretical plate in many separation processes is a hypothetical zone or stage in which two phases, such as the liquid and vapor phases of a substance, establish an equilibrium with each other. Such equilibrium stages may also be referred to as ...
. Hence, a distillation tower needs more trays than the number of theoretical vapor–liquid equilibrium stages. A variety of models have been postulated to estimate tray efficiencies.
In modern industrial uses, a packing material is used in the column instead of trays when low pressure drops across the column are required. Other factors that favor packing are: vacuum systems, smaller diameter columns, corrosive systems, systems prone to foaming, systems requiring low liquid holdup, and batch distillation. Conversely, factors that favor plate columns are: presence of solids in feed, high liquid rates, large column diameters, complex columns, columns with wide feed composition variation, columns with a chemical reaction, absorption columns, columns limited by foundation weight tolerance, low liquid rate, large turn-down ratio and those processes subject to process surges.
 This packing material can either be random or dumped packing ( wide) such as Raschig rings or structured sheet metal. Liquids tend to wet the surface of the packing and the vapors pass across this wetted surface, where
This packing material can either be random or dumped packing ( wide) such as Raschig rings or structured sheet metal. Liquids tend to wet the surface of the packing and the vapors pass across this wetted surface, where mass transfer
Mass transfer is the net movement of mass from one location (usually meaning stream, phase, fraction, or component) to another. Mass transfer occurs in many processes, such as absorption, evaporation, drying, precipitation, membrane filtra ...
takes place. Unlike conventional tray distillation in which every tray represents a separate point of vapor–liquid equilibrium, the vapor–liquid equilibrium curve in a packed column is continuous. However, when modeling packed columns, it is useful to compute a number of "theoretical stages" to denote the separation efficiency of the packed column with respect to more traditional trays. Differently shaped packings have different surface areas and void space between packings. Both these factors affect packing performance.
Another factor in addition to the packing shape and surface area that affects the performance of random or structured packing is the liquid and vapor distribution entering the packed bed. The number of theoretical stages required to make a given separation is calculated using a specific vapor to liquid ratio. If the liquid and vapor are not evenly distributed across the superficial tower area as it enters the packed bed, the liquid to vapor ratio will not be correct in the packed bed and the required separation will not be achieved. The packing will appear to not be working properly. The height equivalent to a theoretical plate (HETP) will be greater than expected. The problem is not the packing itself but the mal-distribution of the fluids entering the packed bed. Liquid mal-distribution is more frequently the problem than vapor. The design of the liquid distributors used to introduce the feed and reflux to a packed bed is critical to making the packing perform to it maximum efficiency. Methods of evaluating the effectiveness of a liquid distributor to evenly distribute the liquid entering a packed bed can be found in references.[Moore, F., Rukovena, F. (August 1987) ''Random Packing, Vapor and Liquid Distribution: Liquid and gas distribution in commercial packed towers'', Chemical Plants & Processing, Edition Europe, pp. 11–15]
Multi-effect distillation
The goal of multi-effect distillation is to increase the energy efficiency Energy efficiency may refer to:
* Energy efficiency (physics), the ratio between the useful output and input of an energy conversion process
** Electrical efficiency, useful power output per electrical power consumed
** Mechanical efficiency, a rat ...
of the process, for use in desalination, or in some cases one stage in the production of ultrapure water
Ultrapure water (UPW), high-purity water or highly purified water (HPW) is water that has been purified to uncommonly stringent specifications. Ultrapure water is a term commonly used in manufacturing to emphasize the fact that the water is treated ...
. The number of effects is inversely proportional to the kW·h/m3 of water recovered figure and refers to the volume of water recovered per unit of energy compared with single-effect distillation. One effect is roughly 636 kW·h/m3:
* Multi-stage flash distillation
Multi-stage flash distillation (MSF) is a water desalination process that distills sea water by flashing a portion of the water into steam in multiple stages of what are essentially countercurrent heat exchangers. Current MSF facilities may h ...
can achieve more than 20 effects with thermal energy input, as mentioned in the article.
* Vapor compression evaporation – Commercial large-scale units can achieve around 72 effects with electrical energy input, according to manufacturers.
There are many other types of multi-effect distillation processes, including one referred to as simply multi-effect distillation (MED), in which multiple chambers, with intervening heat exchangers, are employed.
In food processing
Beverages
Carbohydrate
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' ...
-containing plant materials are allowed to ferment, producing a dilute solution of ethanol in the process. Spirits such as whiskey
Whisky or whiskey is a type of liquor made from Fermentation in food processing, fermented grain mashing, mash. Various grains (which may be Malting, malted) are used for different varieties, including barley, Maize, corn, rye, and wheat. Whisky ...
and rum
Rum is a liquor made by fermenting and then distilling sugarcane molasses or sugarcane juice. The distillate, a clear liquid, is often aged in barrels of oak. Rum originated in the Caribbean in the 17th century, but today it is produced i ...
are prepared by distilling these dilute solutions of ethanol. Components other than ethanol, including water, esters, and other alcohols, are collected in the condensate, which account for the flavor of the beverage. Some of these beverages are then stored in barrels or other containers to acquire more flavor compounds and characteristic flavors.
Gallery
File:Retort-in-operation-early-chemistry.png, Chemistry in its beginnings used retort
In a chemistry laboratory, a retort is a device used for distillation or dry distillation of substances. It consists of a sphere, spherical vessel with a long downward-pointing neck. The liquid to be distilled is placed in the vessel and heat ...
s as laboratory equipment
A laboratory (; ; colloquially lab) is a facility that provides controlled conditions in which scientific or technological research, experiments, and measurement may be performed. Laboratories are found in a variety of settings such as schools, u ...
exclusively for distillation processes.
File:Distillation of dry and oxygen-free toluene.jpg, A simple set-up to distill dry and oxygen-free toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon with the chemical formula , often abbreviated as , where Ph stands for the phenyl group. It is a colorless, water
Water is an inorganic compound with the c ...
.
File:Vacuum Column.png, Diagram of an industrial-scale vacuum distillation column as commonly used in oil refineries
An oil refinery or petroleum refinery is an industrial process plant where petroleum (crude oil) is transformed and refined into products such as gasoline (petrol), diesel fuel, asphalt base, fuel oils, heating oil, kerosene, liquefied pet ...
File:Rotavapor.jpg, A rotary evaporator is able to distill solvents more quickly at lower temperatures through the use of a vacuum
A vacuum (: vacuums or vacua) is space devoid of matter. The word is derived from the Latin adjective (neuter ) meaning "vacant" or "void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressur ...
.
File:Semi-microscale distillation.jpg, Distillation using semi-microscale apparatus. The jointless design eliminates the need to fit pieces together. The pear-shaped flask allows the last drop of residue to be removed, compared with a similarly sized round-bottom flask
Round-bottom flasks (also called round-bottomed flasks or RB flasks) are types of flasks having spherical bottoms used as laboratory glassware, mostly for chemical or biochemical work. They are typically made of glass for chemical inertness; ...
. The small holdup volume prevents losses. A "pig" is used to channel the various distillates into three receiving flasks. If necessary the distillation can be carried out under vacuum using the vacuum adapter at the pig.
See also
* Atmospheric distillation of crude oil
* Clyssus
* Fragrance extraction
* Low-temperature distillation
* Microdistillery
A microdistillery is a small, often boutique-style distillery established to produce beverage grade spirit alcohol in relatively small quantities, usually done in single batches (as opposed to larger distillers' continuous distilling process). W ...
* Sublimation
* Dixon rings
* Random column packing
References
Further reading
*
*
* Needham, Joseph (1980)
''Science and Civilisation in China''
Cambridge University Press. .
External links
*
{{Authority control
Unit operations
Alchemical processes
Separation processes
Laboratory techniques
Phase transitions
Gas technologies
Ancient inventions
 Early evidence of distillation has been found related to
Early evidence of distillation has been found related to  ]
]



 As
As 
 Large scale industrial distillation applications include both batch and continuous fractional, vacuum, azeotropic, extractive, and steam distillation. The most widely used industrial applications of continuous, steady-state fractional distillation are in
Large scale industrial distillation applications include both batch and continuous fractional, vacuum, azeotropic, extractive, and steam distillation. The most widely used industrial applications of continuous, steady-state fractional distillation are in  This packing material can either be random or dumped packing ( wide) such as Raschig rings or structured sheet metal. Liquids tend to wet the surface of the packing and the vapors pass across this wetted surface, where
This packing material can either be random or dumped packing ( wide) such as Raschig rings or structured sheet metal. Liquids tend to wet the surface of the packing and the vapors pass across this wetted surface, where