|
Spinning Band Distillation
Spinning band distillation is a technique used to separate liquid mixtures which are similar in boiling points. When liquids with similar boiling points are distilled, the vapors are mixtures, and not pure compounds. Fractionating columns help separate the mixture by allowing the mixed vapors to cool, condense, and vaporize again in accordance with Raoult's law. With each condensation-vaporization cycles, the vapors are enriched in a certain component. A larger surface area allows more cycles, improving separation. Spinning band distillation takes this concept one step further by using a spinning helical band made of an inert material such as metal or Teflon to push the rising vapors and descending condensate to the sides of the column, coming into close contact with each other. This speeds up equilibration and provides for a greater number of condensation-vaporization cycles. Applications Spinning band distillation may sometimes be used to recycle waste solvents which contain dif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Distillation
Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixture and the condensation of the vapors in a still. Distillation can operate over a wide range of pressures from 0.14 bar (e.g., ethylbenzene/ styrene) to nearly 21 bar (e.g., propylene/propane) and is capable of separating feeds with high volumetric flowrates and various components that cover a range of relative volatilities from only 1.17 ( o-xylene/ m-xylene) to 81.2 (water/ ethylene glycol). Distillation provides a convenient and time-tested solution to separate a diversity of chemicals in a continuous manner with high purity. However, distillation has an enormous environmental footprint, resulting in the consumption of approximately 25% of all industrial energy use. The key issue is that distillation operates based on phase changes, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fractionating Column
A fractionating column or fractional column is equipment used in the distillation of liquid mixtures to separate the mixture into its component parts, or fractions, based on their differences in volatility. Fractionating columns are used in small-scale laboratory distillations as well as large-scale industrial distillations. Laboratory fractionating columns A laboratory fractionating column is a piece of glassware used to separate vaporized mixtures of liquid compounds with close volatility. Most commonly used is either a Vigreux column or a straight column packed with glass beads or metal pieces such as Raschig rings. Fractionating columns help to separate the mixture by allowing the mixed vapors to cool, condense, and vaporize again in accordance with Raoult's law. With each condensation-vaporization cycle, the vapors are enriched in a certain component. A larger surface area allows more cycles, improving separation. This is the rationale for a Vigreux column or a packed fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
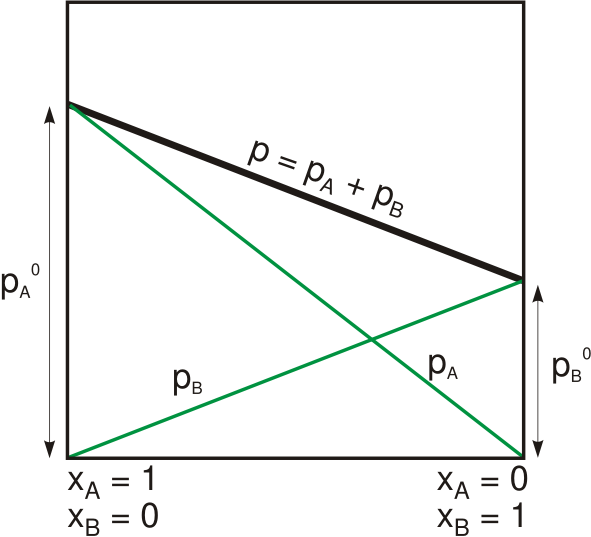
Raoult's Law
Raoult's law ( law) is a relation of physical chemistry, with implications in thermodynamics. Proposed by French chemist François-Marie Raoult in 1887, it states that the partial pressure of each component of an ideal mixture of ''liquids'' is equal to the vapor pressure of the pure component (liquid or solid) multiplied by its mole fraction in the mixture. In consequence, the relative lowering of vapor pressure of a dilute solution of nonvolatile solute is equal to the mole fraction of solute in the solution. Mathematically, Raoult's law for a single component in an ideal solution is stated as : p_i = p_i^\star x_i where p_i is the partial pressure of the component i in the gaseous mixture above the solution, p_i^\star is the equilibrium vapor pressure of the pure component i, and x_i is the mole fraction of the component i in the liquid or solid solution. Where two volatile liquids A and B are mixed with each other to form a solution, the vapor phase consists of both compo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Teflon
Polytetrafluoroethylene (PTFE) is a synthetic fluoropolymer of tetrafluoroethylene, and has numerous applications because it is chemically inert. The commonly known brand name of PTFE-based composition is Teflon by Chemours, a spin-off from DuPont, which originally invented the compound in 1938. Polytetrafluoroethylene is a fluorocarbon solid, as it is a high- molecular-weight polymer consisting wholly of carbon and fluorine. PTFE is hydrophobic: neither water nor water-containing substances wet PTFE, as fluorocarbons exhibit only small London dispersion forces due to the low electric polarizability of fluorine. PTFE has one of the lowest coefficients of friction of any solid. Polytetrafluoroethylene is used as a non-stick coating for pans and other cookware. It is non-reactive, partly because of the strength of carbon–fluorine bonds, so it is often used in containers and pipework for reactive and corrosive chemicals. When used as a lubricant, PTFE reduces fric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken or new bonds formed or both. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, usin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spinning Cone
Spinning cone columns are used in a form of low temperature vacuum steam distillation to gently extract volatile chemicals from liquid foodstuffs while minimising the effect on the taste of the product. For instance, the columns can be used to remove some of the alcohol from wine, to remove "off" smells from cream, and to capture aroma compounds that would otherwise be lost in coffee processing. Mechanism The columns are made of stainless steel. Conical vanes are attached alternately to the wall of the column and to a central rotating shaft. The product is poured in at the top under vacuum, and steam is pumped into the column from below. The vanes provide a large surface area over which volatile compounds can evaporate into the steam, and the rotation ensures a thin layer of the product is constantly moved over the moving cone. It typically takes 20 seconds for the liquid to move through the column, and industrial columns might process . The temperature and pressure can be adjus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Distillation
Distillation, also classical distillation, is the process of separating the component substances of a liquid mixture of two or more chemically discrete substances; the separation process is realized by way of the selective boiling of the mixture and the condensation of the vapors in a still. Distillation can operate over a wide range of pressures from 0.14 bar (e.g., ethylbenzene/ styrene) to nearly 21 bar (e.g., propylene/propane) and is capable of separating feeds with high volumetric flowrates and various components that cover a range of relative volatilities from only 1.17 ( o-xylene/ m-xylene) to 81.2 (water/ ethylene glycol). Distillation provides a convenient and time-tested solution to separate a diversity of chemicals in a continuous manner with high purity. However, distillation has an enormous environmental footprint, resulting in the consumption of approximately 25% of all industrial energy use. The key issue is that distillation operates based on phase changes, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Separation Processes
A separation process is a method that converts a mixture or a solution of chemical substances into two or more distinct product mixtures, a scientific process of separating two or more substances in order to obtain purity. At least one product mixture from the separation is enriched in one or more of the source mixture's constituents. In some cases, a separation may fully divide the mixture into pure constituents. Separations exploit differences in chemical properties or physical properties (such as size, shape, charge, mass, density, or chemical affinity) between the constituents of a mixture. Processes are often classified according to the particular properties they exploit to achieve separation. If no single difference can be used to accomplish the desired separation, multiple operations can often be combined to achieve the desired end. Different processes are also sometimes categorized by their separating agent, i.e. ''mass separating agents'' or ''energy separating agents ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Laboratory Equipment
A laboratory (; ; colloquially lab) is a facility that provides controlled conditions in which scientific or technological research, experiments, and measurement may be performed. Laboratories are found in a variety of settings such as schools, universities, privately owned research institutions, corporate research and testing facilities, government regulatory and forensic investigation centers, physicians' offices, clinics, hospitals, regional and national referral centers, and even occasionally personal residences. Overview The organisation and contents of laboratories are determined by the differing requirements of the specialists working within. A physics laboratory might contain a particle accelerator or vacuum chamber, while a metallurgy laboratory could have apparatus for casting or refining metals or for testing their strength. A chemist or biologist might use a wet laboratory, while a psychologist's laboratory might be a room with one-way mirrors and hidden cameras in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |