Autophagy on:
[Wikipedia]
[Google]
[Amazon]
 Autophagy (or autophagocytosis; from the
Autophagy (or autophagocytosis; from the
 Text was copied from this source, which is available under
Text was copied from this source, which is available under
Creative Commons Attribution 4.0 International License
On the other hand, bacterial proteins from various pathogenic genera are also able to modulate autophagy. There are genus-specific patterns in the phases of autophagy that are potentially regulated by a given pathogen group. Some autophagy phases can only be modulated by particular pathogens, while some phases are modulated by multiple pathogen genera. Some of the interplay-related bacterial proteins have proteolytic and post-translational activity such as phosphorylation and ubiquitination and can interfere with the activity of autophagy proteins.
''Autophagy'', a journal produced by Landes Bioscience and edited by DJ Klionsky
LongevityMeme entry describing PubMed article on the effects of autophagy and lifespan
''HADb'', a Human Autophagy dedicated Database
''Autophagy DB'', an autophagy database that covers all eukaryotes
* ttp://well.blogs.nytimes.com/2012/02/01/exercise-as-housecleaning-for-the-body Exercise as Housecleaning for the Body
The AIM center
{{DEFAULTSORT:Autophagy (Cellular) Cellular processes Programmed cell death Immunology Cell death
 Autophagy (or autophagocytosis; from the
Autophagy (or autophagocytosis; from the Ancient Greek
Ancient Greek includes the forms of the Greek language used in ancient Greece and the ancient world from around 1500 BC to 300 BC. It is often roughly divided into the following periods: Mycenaean Greek (), Dark Ages (), the Archaic p ...
, , meaning "self-devouring" and , , meaning "hollow") is the natural, conserved degradation of the cell that removes unnecessary or dysfunctional components through a lysosome-dependent regulated mechanism. It allows the orderly degradation and recycling of cellular components. Although initially characterized as a primordial degradation pathway induced to protect against starvation, it has become increasingly clear that autophagy also plays a major role in the homeostasis
In biology, homeostasis (British English, British also homoeostasis) Help:IPA/English, (/hɒmɪə(ʊ)ˈsteɪsɪs/) is the state of steady internal, physics, physical, and chemistry, chemical conditions maintained by organism, living systems. Thi ...
of non-starved cells. Defects in autophagy have been linked to various human diseases, including neurodegeneration and cancer, and interest in modulating autophagy as a potential treatment for these diseases has grown rapidly.
Four forms of autophagy have been identified: macroautophagy, microautophagy, chaperone-mediated autophagy (CMA), and crinophagy. In macroautophagy (the most thoroughly researched form of autophagy), cytoplasm
In cell biology, the cytoplasm is all of the material within a eukaryotic cell, enclosed by the cell membrane, except for the cell nucleus. The material inside the nucleus and contained within the nuclear membrane is termed the nucleoplasm. ...
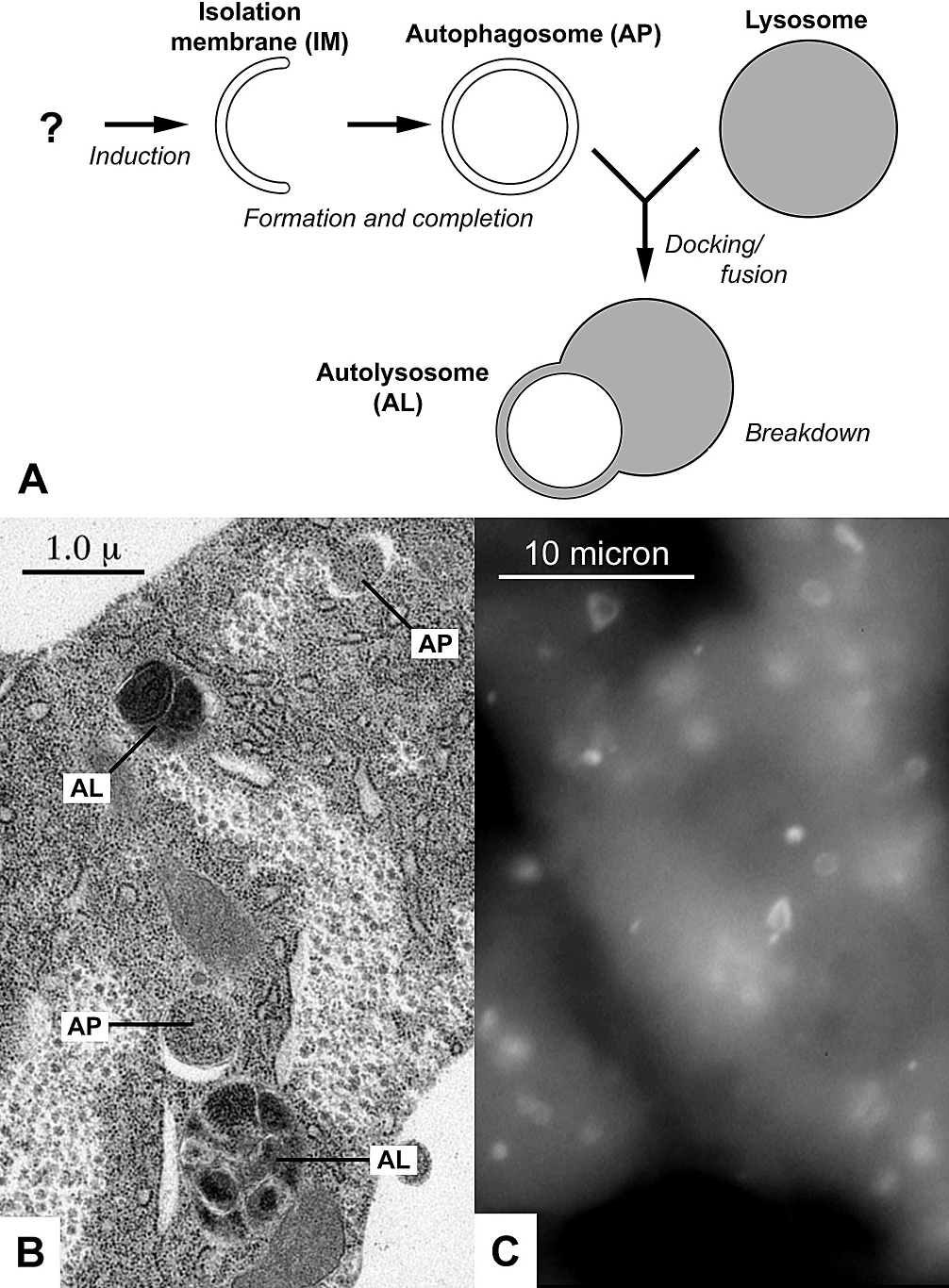
ic components (like mitochondria) are targeted and isolated from the rest of the cell within a double-membrane vesicle known as an autophagosome, which, in time, fuses with an available lysosome
A lysosome () is a membrane-bound organelle found in many animal cells. They are spherical vesicles that contain hydrolytic enzymes that can break down many kinds of biomolecules. A lysosome has a specific composition, of both its membrane ...
, bringing its specialty process of waste management and disposal; and eventually the contents of the vesicle (now called an autolysosome) are degraded and recycled. In crinophagy (the least well-known and researched form of autophagy), unnecessary secretory granules are degraded and recycled.
In disease, autophagy has been seen as an adaptive response to stress, promoting survival of the cell; but in other cases, it appears to promote cell death and morbidity. In the extreme case of starvation, the breakdown of cellular components promotes cellular survival by maintaining cellular energy levels.
The word "autophagy" was in existence and frequently used from the middle of the 19th century. In its present usage, the term autophagy was coined by Belgian biochemist Christian de Duve in 1963 based on his discovery of the functions of lysosome. The identification of autophagy-related genes in yeast in the 1990s allowed researchers to deduce the mechanisms of autophagy, which eventually led to the award of the 2016 Nobel Prize in Physiology or Medicine
The Nobel Prize in Physiology or Medicine ( sv, Nobelpriset i fysiologi eller medicin) is awarded yearly by the Nobel Assembly at the Karolinska Institute, Nobel Assembly at the Karolinska Institute for outstanding discoveries in physiology or ...
to Japanese researcher Yoshinori Ohsumi.
History
Autophagy was first observed by Keith R. Porter and his student Thomas Ashford at the Rockefeller Institute. In January 1962 they reported an increased number of lysosomes in rat liver cells after the addition of glucagon, and that some displaced lysosomes towards the centre of the cell contained other cell organelles such as mitochondria. They called this autolysis after Christian de Duve and Alex B. Novikoff. However Porter and Ashford wrongly interpreted their data as lysosome formation (ignoring the pre-existing organelles). Lysosomes could not be cell organelles, but part ofcytoplasm
In cell biology, the cytoplasm is all of the material within a eukaryotic cell, enclosed by the cell membrane, except for the cell nucleus. The material inside the nucleus and contained within the nuclear membrane is termed the nucleoplasm. ...
such as mitochondria, and that hydrolytic enzymes were produced by microbodies. In 1963 Hruban, Spargo and colleagues published a detailed ultrastructural description of "focal cytoplasmic degradation", which referenced a 1955 German study of injury-induced sequestration. Hruban, Spargo and colleagues recognized three continuous stages of maturation of the sequestered cytoplasm to lysosomes, and that the process was not limited to injury states that functioned under physiological conditions for "reutilization of cellular materials", and the "disposal of organelles" during differentiation. Inspired by this discovery, de Duve christened the phenomena "autophagy". Unlike Porter and Ashford, de Duve conceived the term as a part of lysosomal function while describing the role of glucagon as a major inducer of cell degradation in the liver. With his student Russell Deter, he established that lysosomes are responsible for glucagon-induced autophagy. This was the first time the fact that lysosomes are the sites of intracellular autophagy was established.
In the 1990s several groups of scientists independently discovered autophagy-related genes using the budding yeast. Notably, Yoshinori Ohsumi and Michael Thumm examined starvation-induced non-selective autophagy; in the meantime, Daniel J. Klionsky
Daniel Jay Klionsky (born 1958) is an American biochemist and molecular biologist. He is the Alexander G. Ruthven Professor of Life Sciences and professor of molecular, cellular, and developmental biology at the University of Michigan. As a cell b ...
discovered the cytoplasm-to-vacuole targeting (CVT) pathway, which is a form of selective autophagy. They soon found that they were in fact looking at essentially the same pathway, just from different angles. Initially, the genes discovered by these and other yeast groups were given different names (APG, AUT, CVT, GSA, PAG, PAZ, and PDD). A unified nomenclature was advocated in 2003 by the yeast researchers to use ATG to denote autophagy genes. The 2016 Nobel Prize in Physiology or Medicine was awarded to Yoshinori Ohsumi, although some have pointed out that the award could have been more inclusive.
The field of autophagy research experienced accelerated growth at the turn of the 21st century. Knowledge of ATG genes provided scientists more convenient tools to dissect functions of autophagy in human health and disease. In 1999, a landmark discovery connecting autophagy with cancer was published by Beth Levine's group. To this date, relationship between cancer and autophagy continues to be a main theme of autophagy research. The roles of autophagy in neurodegeneration and immune defense also received considerable attention. In 2003, the first Gordon Research Conference on autophagy was held at Waterville. In 2005, Daniel J Klionsky launched ''Autophagy'', a scientific journal dedicated to this field. The first Keystone Symposia Conference on autophagy was held in 2007 at Monterey. In 2008, Carol A Mercer created a BHMT fusion protein (GST-BHMT), which showed starvation-induced site-specific fragmentation in cell lines. The degradation of betaine homocysteine methyltransferase (BHMT), a metabolic enzyme, could be used to assess autophagy flux in mammalian cells.
Macro, micro, and Chaperone mediated autophagy are mediated by autophagy-related genes and their associated enzymes. Macroautophagy is then divided into bulk and selective autophagy. In the selective autophagy is the autophagy of organelles; mitophagy, lipophagy, pexophagy, chlorophagy, ribophagy and others.
Macroautophagy is the main pathway, used primarily to eradicate damaged cell organelle
In cell biology, an organelle is a specialized subunit, usually within a cell, that has a specific function. The name ''organelle'' comes from the idea that these structures are parts of cells, as organs are to the body, hence ''organelle,'' t ...
s or unused proteins. First the phagophore engulfs the material that needs to be degraded, which forms a double membrane
A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Membranes can be generally classified into synthetic membranes and biological membranes. ...
known as an autophagosome, around the organelle marked for destruction. The autophagosome then travels through the cytoplasm of the cell to a lysosome in mammals, or vacuoles in yeast and plants, and the two organelles fuse. Within the lysosome/vacuole, the contents of the autophagosome are degraded via acidic lysosomal hydrolase.
Microautophagy, on the other hand, involves the direct engulfment of cytoplasmic material into the lysosome. This occurs by invagination, meaning the inward folding of the lysosomal membrane, or cellular protrusion.
Chaperone-mediated autophagy, or CMA, is a very complex and specific pathway, which involves the recognition by the hsc70-containing complex. This means that a protein must contain the recognition site for this hsc70 complex which will allow it to bind to this chaperone, forming the CMA- substrate/chaperone complex. This complex then moves to the lysosomal membrane-bound protein that will recognise and bind with the CMA receptor. Upon recognition, the substrate protein gets unfolded and it is translocated across the lysosome membrane with the assistance of the lysosomal hsc70 chaperone. CMA is significantly different from other types of autophagy because it translocates protein material in a one by one manner, and it is extremely selective about what material crosses the lysosomal barrier.
Mitophagy Mitophagy is the selective degradation of mitochondria by autophagy. It often occurs to defective mitochondria following damage or stress. The process of mitophagy was first described over a hundred years ago by Margaret Reed Lewis and Warren Har ...
is the selective degradation of mitochondria by autophagy. It often occurs to defective mitochondria following damage or stress. Mitophagy promotes the turnover of mitochondria and prevents the accumulation of dysfunctional mitochondria which can lead to cellular degeneration. It is mediated by Atg32 (in yeast) and NIX and its regulator BNIP3 in mammals. Mitophagy is regulated by PINK1 and parkin proteins. The occurrence of mitophagy is not limited to the damaged mitochondria but also involves undamaged ones.
Lipophagy is the degradation of lipids by autophagy, a function which has been shown to exist in both animal and fungal cells. The role of lipophagy in plant cells, however, remains elusive. In lipophagy the target are lipid structures called lipid droplets (LDs), spheric "organelles" with a core of mainly triacylglycerols (TAGs) and a unilayer of phospholipids and membrane proteins. In animal cells the main lipophagic pathway is via the engulfment of LDs by the phagophore, macroautophagy. In fungal cells on the other hand microplipophagy constitutes the main pathway and is especially well studied in the budding yeast ''Saccharomyces cerevisiae
''Saccharomyces cerevisiae'' () (brewer's yeast or baker's yeast) is a species of yeast (single-celled fungus microorganisms). The species has been instrumental in winemaking, baking, and brewing since ancient times. It is believed to have been o ...
'.'' Lipophagy was first discovered in mice and published 2009.
Targeted interplay between bacterial pathogens and host autophagy
Autophagy targets genus-specific proteins, so orthologous proteins which share sequence homology with each other are recognized as substrates by a particular autophagy targeting protein. There exists a complementarity of autophagy targeting proteins which potentially increase infection risk upon mutation. The lack of overlap among the targets of the 3 autophagy proteins and the large overlap in terms of the genera show that autophagy could target different sets of bacterial proteins from a same pathogen. On one hand, the redundancy in targeting a same genera is beneficial for robust pathogen recognition. But, on the other hand, the complementarity in the specific bacterial proteins could make the host more susceptible to chronic disorders and infections if the gene encoding one of the autophagy targeting proteins becomes mutated, and the autophagy system is overloaded or suffers other malfunctions. Moreover, autophagy targets virulence factors and virulence factors responsible for more general functions such as nutrient acquisition and motility are recognized by multiple autophagy targeting proteins. And the specialized virulence factors such as autolysins, and iron sequestering proteins are potentially recognized uniquely by a single autophagy targeting protein. The autophagy proteins CALCOCO2/NDP52 and MAP1LC3/LC3 may have evolved specifically to target pathogens or pathogenic proteins for autophagic degradation. While SQSTM1/p62 targets more generic bacterial proteins containing a target motif but not related to virulence.Creative Commons Attribution 4.0 International License
On the other hand, bacterial proteins from various pathogenic genera are also able to modulate autophagy. There are genus-specific patterns in the phases of autophagy that are potentially regulated by a given pathogen group. Some autophagy phases can only be modulated by particular pathogens, while some phases are modulated by multiple pathogen genera. Some of the interplay-related bacterial proteins have proteolytic and post-translational activity such as phosphorylation and ubiquitination and can interfere with the activity of autophagy proteins.
Molecular biology
Autophagy is executed by autophagy-related (Atg) genes. Prior to 2003, ten or more names were used, but after this point a unified nomenclature was devised by fungal autophagy researchers. Atg or ATG stands for autophagy related. It does not specify gene or a protein. The first autophagy genes were identified by genetic screens conducted in ''Saccharomyces cerevisiae''. Following their identification those genes were functionally characterized and their orthologs in a variety of different organisms were identified and studied. Today, thirty-six Atg proteins have been classified as especially important for autophagy, of which 18 belong to the core machinery In mammals,amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha ...
sensing and additional signals such as growth factor
A growth factor is a naturally occurring substance capable of stimulating cell proliferation, wound healing, and occasionally cellular differentiation. Usually it is a secreted protein or a steroid hormone. Growth factors are important for r ...
s and reactive oxygen species
In chemistry, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (). Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen.
The reduction of molecular oxygen ...
regulate the activity of the protein kinases
In biochemistry, a kinase () is an enzyme that catalyzes the transfer of phosphate groups from high-energy, phosphate-donating molecules to specific substrates. This process is known as phosphorylation, where the high-energy ATP molecule donat ...
mTOR and AMPK. These two kinases regulate autophagy through inhibitory phosphorylation of the Unc-51-like kinases ULK1 and ULK2 (mammalian homologues of Atg1). Induction of autophagy results in the dephosphorylation and activation of the ULK kinases. ULK is part of a protein complex containing Atg13, Atg101 and FIP200. ULK phosphorylates and activates Beclin-1
Beclin-1 is a protein that in humans is encoded by the ''BECN1'' gene. Beclin-1 is a mammalian ortholog of the yeast autophagy-related gene 6 (Atg6) and BEC-1 in the C. elegans nematode. This protein interacts with either BCL-2 or PI3k cla ...
(mammalian homologue of Atg6), which is also part of a protein complex. The autophagy-inducible Beclin-1 complex contains the proteins PIK3R4(p150), Atg14L and the class III phosphatidylinositol 3-phosphate kinase (PI(3)K) Vps34. The active ULK and Beclin-1 complexes re-localize to the site of autophagosome initiation, the phagophore, where they both contribute to the activation of downstream autophagy components.
Once active, VPS34 phosphorylates the lipid
Lipids are a broad group of naturally-occurring molecules which includes fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids incl ...
phosphatidylinositol to generate phosphatidylinositol 3-phosphate (PtdIns(3)P) on the surface of the phagophore. The generated PtdIns(3)P is used as a docking point for proteins harboring a PtdIns(3)P binding motif. WIPI2
WD repeat domain phosphoinositide-interacting protein 2 is a protein that in humans is encoded by the ''WIPI2'' gene.
Function
WD40 repeat proteins are key components of many essential biologic functions. They regulate the assembly of multi ...
, a PtdIns(3)P binding protein of the WIPI (WD-repeat protein interacting with phosphoinositides) protein family, was recently shown to physically bind Atg16L1
Autophagy related 16 like 1 is a protein that in humans is encoded by the ''ATG16L1'' gene. This protein is characterized as a subunit of the autophagy-related ATG12-ATG5/ATG16 complex and is essentially important for the LC3 (ATG8) lipidation and ...
. Atg16L1 is a member of an E3-like protein complex involved in one of two ubiquitin-like conjugation systems essential for autophagosome formation. The FIP200 cis-Golgi-derived membranes fuse with ATG16L1-positive endosomal membranes to form the prophagophore termed HyPAS (hybrid pre-autophagosomal structure). ATG16L1 binding to WIPI2 mediates ATG16L1's activity. This leads to downstream conversion of prophagophore into ATG8-positive phagophore via a ubiquitin-like conjugation system.
The first of the two ubiquitin-like conjugation systems involved in autophagy covalently binds the ubiquitin-like protein Atg12 to Atg5. The resulting conjugate protein then binds Atg16L1
Autophagy related 16 like 1 is a protein that in humans is encoded by the ''ATG16L1'' gene. This protein is characterized as a subunit of the autophagy-related ATG12-ATG5/ATG16 complex and is essentially important for the LC3 (ATG8) lipidation and ...
to form an E3-like complex which functions as part of the second ubiquitin-like conjugation system. This complex binds and activates Atg3, which covalently attaches mammalian homologues of the ubiquitin-like yeast protein ATG8 ( LC3A-C, GATE16, and GABARAPL1-3), the most studied being LC3 proteins, to the lipid phosphatidylethanolamine (PE) on the surface of autophagosomes. Lipidated LC3 contributes to the closure of autophagosomes, and enables the docking of specific cargos and adaptor proteins such as Sequestosome-1/p62 P62 may refer to:
Naval vessels
* , a submarine of the Royal Navy
* , a corvette of the Indian Navy
* , an offshore patrol vessel of the Irish Naval Service
* P-62 ''Explorer'', a fictional icebreaker in the 2019 Indian film ''War''
Other uses
* ...
. The completed autophagosome then fuses with a lysosome
A lysosome () is a membrane-bound organelle found in many animal cells. They are spherical vesicles that contain hydrolytic enzymes that can break down many kinds of biomolecules. A lysosome has a specific composition, of both its membrane ...
through the actions of multiple proteins, including SNAREs and UVRAG. Following the fusion LC3 is retained on the vesicle's inner side and degraded along with the cargo, while the LC3 molecules attached to the outer side are cleaved off by Atg4 and recycled. The contents of the autolysosome are subsequently degraded and their building blocks are released from the vesicle through the action of permeases.
Sirtuin 1 (SIRT1) stimulates autophagy by preventing acetylation of proteins (via deacetylation) required for autophagy as demonstrated in cultured cells and embryonic and neonatal tissues. This function provides a link between sirtuin expression and the cellular response to limited nutrients due to caloric restriction.
Functions
Nutrient starvation
Autophagy has roles in various cellular functions. One particular example is in yeasts, where the nutrient starvation induces a high level of autophagy. This allows unneeded proteins to be degraded and the amino acids recycled for the synthesis of proteins that are essential for survival. In higher eukaryotes, autophagy is induced in response to the nutrient depletion that occurs in animals at birth after severing off the trans-placental food supply, as well as that of nutrient starved cultured cells and tissues. Mutant yeast cells that have a reduced autophagic capability rapidly perish in nutrition-deficient conditions. Studies on the ''apg'' mutants suggest that autophagy via autophagic bodies is indispensable for protein degradation in the vacuoles under starvation conditions, and that at least 15 APG genes are involved in autophagy in yeast. A gene known as ATG7 has been implicated in nutrient-mediated autophagy, as mice studies have shown that starvation-induced autophagy was impaired in ''atg7''-deficient mice.Infection
Vesicular stomatitis virus is believed to be taken up by the autophagosome from the cytosol and translocated to theendosome
Endosomes are a collection of intracellular sorting organelles in eukaryotic cells. They are parts of endocytic membrane transport pathway originating from the trans Golgi network. Molecules or ligands internalized from the plasma membrane c ...
s where detection takes place by a pattern recognition receptor
Pattern recognition receptors (PRRs) play a crucial role in the proper function of the innate immune system. PRRs are germline-encoded host sensors, which detect molecules typical for the pathogens. They are proteins expressed, mainly, by cells of ...
called toll-like receptor 7, detecting single stranded RNA
Ribonucleic acid (RNA) is a polymeric molecule essential in various biological roles in coding, decoding, regulation and expression of genes. RNA and deoxyribonucleic acid ( DNA) are nucleic acids. Along with lipids, proteins, and carbohydra ...
. Following activation of the toll-like receptor, intracellular signaling cascades are initiated, leading to induction of interferon and other antiviral cytokine
Cytokines are a broad and loose category of small proteins (~5–25 kDa) important in cell signaling. Cytokines are peptides and cannot cross the lipid bilayer of cells to enter the cytoplasm. Cytokines have been shown to be involved in a ...
s. A subset of viruses and bacteria subvert the autophagic pathway to promote their own replication. Galectin-8
Galectin-8 is a protein of the galectin family that in humans is encoded by the ''LGALS8'' gene.
Function
This gene encodes a member of the galectin family. Galectins are beta-galactoside-binding animal lectins with conserved carbohydrate recog ...
has recently been identified as an intracellular "danger receptor", able to initiate autophagy against intracellular pathogens. When galectin-8 binds to a damaged vacuole, it recruits an autophagy adaptor such as NDP52 leading to the formation of an autophagosome and bacterial degradation.
Repair mechanism
Autophagy degrades damaged organelles, cell membranes and proteins, and insufficient autophagy is thought to be one of the main reasons for the accumulation of damaged cells and aging. Autophagy and autophagy regulators are involved in response to lysosomal damage, often directed by galectins such as galectin-3 andgalectin-8
Galectin-8 is a protein of the galectin family that in humans is encoded by the ''LGALS8'' gene.
Function
This gene encodes a member of the galectin family. Galectins are beta-galactoside-binding animal lectins with conserved carbohydrate recog ...
.
Programmed cell death
One of the mechanisms ofprogrammed cell death
Programmed cell death (PCD; sometimes referred to as cellular suicide) is the death of a cell (biology), cell as a result of events inside of a cell, such as apoptosis or autophagy. PCD is carried out in a biological process, which usually confers ...
(PCD) is associated with the appearance of autophagosomes and depends on autophagy proteins. This form of cell death most likely corresponds to a process that has been morphologically defined as autophagic PCD. One question that constantly arises, however, is whether autophagic activity in dying cells is the cause of death or is actually an attempt to prevent it. Morphological and histochemical studies have not so far proved a causative relationship between the autophagic process and cell death. In fact, there have recently been strong arguments that autophagic activity in dying cells might actually be a survival mechanism. Studies of the metamorphosis of insects have shown cells undergoing a form of PCD that appears distinct from other forms; these have been proposed as examples of autophagic cell death. Recent pharmacological and biochemical studies have proposed that survival and lethal autophagy can be distinguished by the type and degree of regulatory signaling during stress particularly after viral infection. Although promising, these findings have not been examined in non-viral systems.
Exercise
Autophagy is essential for basalhomeostasis
In biology, homeostasis (British English, British also homoeostasis) Help:IPA/English, (/hɒmɪə(ʊ)ˈsteɪsɪs/) is the state of steady internal, physics, physical, and chemistry, chemical conditions maintained by organism, living systems. Thi ...
; it is also extremely important in maintaining muscle
Skeletal muscles (commonly referred to as muscles) are Organ (biology), organs of the vertebrate muscular system and typically are attached by tendons to bones of a skeleton. The muscle cells of skeletal muscles are much longer than in the other ...
homeostasis during physical exercise. Autophagy at the molecular level is only partially understood. A study of mice shows that autophagy is important for the ever-changing demands of their nutritional and energy needs, particularly through the metabolic pathways of protein catabolism. In a 2012 study conducted by the University of Texas Southwestern Medical Center in Dallas
Dallas () is the List of municipalities in Texas, third largest city in Texas and the largest city in the Dallas–Fort Worth metroplex, the List of metropolitan statistical areas, fourth-largest metropolitan area in the United States at 7.5 ...
, mutant mice (with a knock-in mutation of BCL2 phosphorylation sites to produce progeny that showed normal levels of basal autophagy yet were deficient in stress-induced autophagy) were tested to challenge this theory. Results showed that when compared to a control group, these mice illustrated a decrease in endurance and an altered glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, usi ...
metabolism during acute exercise.
Another study demonstrated that skeletal muscle
Skeletal muscles (commonly referred to as muscles) are organs of the vertebrate muscular system and typically are attached by tendons to bones of a skeleton. The muscle cells of skeletal muscles are much longer than in the other types of m ...
fibers of collagen VI in knockout mice showed signs of degeneration due to an insufficiency of autophagy which led to an accumulation of damaged mitochondria and excessive cell death
Cell death is the event of a biological cell ceasing to carry out its functions. This may be the result of the natural process of old cells dying and being replaced by new ones, as in programmed cell death, or may result from factors such as di ...
. Exercise-induced autophagy was unsuccessful however; but when autophagy was induced artificially post-exercise, the accumulation of damaged organelles in collagen VI deficient muscle fibres was prevented and cellular homeostasis was maintained. Both studies demonstrate that autophagy induction may contribute to the beneficial metabolic effects of exercise and that it is essential in the maintaining of muscle homeostasis during exercise, particularly in collagen VI fibers.
Work at the Institute for Cell Biology, University of Bonn, showed that a certain type of autophagy, i.e. chaperone-assisted selective autophagy (CASA) Chaperone-assisted selective autophagy is a cellular process for the selective, ubiquitin-dependent degradation of chaperone-bound proteins in lysosomes.
Autophagy (Greek: ‘self-eating’) was initially identified as a catabolic process for the ...
, is induced in contracting muscles and is required for maintaining the muscle sarcomere under mechanical tension. The CASA chaperone complex recognizes mechanically damaged cytoskeleton components and directs these components through a ubiquitin-dependent autophagic sorting pathway to lysosomes for disposal. This is necessary for maintaining muscle activity.
Osteoarthritis
Because autophagy decreases with age and age is a major risk factor forosteoarthritis
Osteoarthritis (OA) is a type of degenerative joint disease that results from breakdown of joint cartilage and underlying bone which affects 1 in 7 adults in the United States. It is believed to be the fourth leading cause of disability in the ...
, the role of autophagy in the development of this disease is suggested. Proteins involved in autophagy are reduced with age in both human and mouse articular cartilage. Mechanical injury to cartilage explants in culture also reduced autophagy proteins. Autophagy is constantly activated in normal cartilage but it is compromised with age and precedes cartilage cell death and structural damage. Thus autophagy is involved in a normal protective process (chondroprotection A chondroprotective compound is a specific compound or chemical that delays progressive joint space narrowing characteristic of arthritis and improves the biomechanics of articular joints by protecting chondrocytes. These agents perform various fun ...
) in the joint.
Cancer
Cancer often occurs when several different pathways that regulate cell differentiation are disturbed. Autophagy plays an important role in cancer – both in protecting against cancer as well as potentially contributing to the growth of cancer.Furuya, N., Liang, X.H., and Levin, B. 2004. Autophagy and cancer. In Autophagy. D.J. Klionsky editor. Landes Bioscience. Georgetown, Texas, USA. 244-253. Autophagy can contribute to cancer by promoting survival of tumor cells that have been starved, or that degrade apoptotic mediators through autophagy: in such cases, use of inhibitors of the late stages of autophagy (such as chloroquine), on the cells that use autophagy to survive, increases the number of cancer cells killed by antineoplastic drugs. The role of autophagy in cancer is one that has been highly researched and reviewed. There is evidence that emphasizes the role of autophagy as both a tumor suppressor and a factor in tumor cell survival. Recent research has shown, however, that autophagy is more likely to be used as a tumor suppressor according to several models.Tumor suppressor
Several experiments have been done with mice and varying Beclin1, a protein that regulates autophagy. When the Beclin1 gene was altered to be heterozygous (Beclin 1+/-), the mice were found to be tumor-prone. However, when Beclin1 was overexpressed, tumor development was inhibited. Care should be exercised when interpreting phenotypes of beclin mutants and attributing the observations to a defect in autophagy, however: Beclin1 is generally required for phosphatidylinositol 3- phosphate production and as such it affects numerous lysosomal and endosomal functions, including endocytosis and endocytic degradation of activated growth factor receptors. In support of the possibility that Beclin1 affects cancer development through an autophagy-independent pathway is the fact that core autophagy factors which are not known to affect other cellular processes and are definitely not known to affect cell proliferation and cell death, such as Atg7 or Atg5, show a much different phenotype when the respective gene is knocked out, which does not include tumor formation. In addition, full knockout of Beclin1 is embryonic lethal whereas knockout of Atg7 or Atg5 is not. Necrosis and chronic inflammation also has been shown to be limited through autophagy which helps protect against the formation of tumor cells.Mechanism of cell death
Cells that undergo an extreme amount of stress experience cell death either through apoptosis ornecrosis
Necrosis () is a form of cell injury which results in the premature death of cells in living tissue by autolysis. Necrosis is caused by factors external to the cell or tissue, such as infection, or trauma which result in the unregulated dig ...
. Prolonged autophagy activation leads to a high turnover rate of proteins and organelles. A high rate above the survival threshold may kill cancer cells with a high apoptotic threshold. This technique can be utilized as a therapeutic cancer treatment.
Tumor cell survival
Alternatively, autophagy has also been shown to play a large role in tumor cell survival. In cancerous cells, autophagy is used as a way to deal with stress on the cell. Induction of autophagy by miRNA-4673, for example, is a pro-survival mechanism that improves the resistance of cancer cells to radiation. Once these autophagy related genes were inhibited, cell death was potentiated. The increase in metabolic energy is offset by autophagy functions. These metabolic stresses include hypoxia, nutrient deprivation, and an increase in proliferation. These stresses activate autophagy in order to recycle ATP and maintain survival of the cancerous cells. Autophagy has been shown to enable continued growth of tumor cells by maintaining cellular energy production. By inhibiting autophagy genes in these tumors cells, regression of the tumor and extended survival of the organs affected by the tumors were found. Furthermore, inhibition of autophagy has also been shown to enhance the effectiveness of anticancer therapies.Therapeutic target
New developments in research have found that targeted autophagy may be a viable therapeutic solution in fighting cancer. As discussed above, autophagy plays both a role in tumor suppression and tumor cell survival. Thus, the qualities of autophagy can be used as a strategy for cancer prevention. The first strategy is to induce autophagy and enhance its tumor suppression attributes. The second strategy is to inhibit autophagy and thus induce apoptosis. The first strategy has been tested by looking at dose-response anti-tumor effects during autophagy-induced therapies. These therapies have shown that autophagy increases in a dose-dependent manner. This is directly related to the growth of cancer cells in a dose-dependent manner as well. These data support the development of therapies that will encourage autophagy. Secondly, inhibiting the protein pathways directly known to induce autophagy may also serve as an anticancer therapy. The second strategy is based on the idea that autophagy is a protein degradation system used to maintain homeostasis and the findings that inhibition of autophagy often leads to apoptosis. Inhibition of autophagy is riskier as it may lead to cell survival instead of the desired cell death.Negative regulators of autophagy
Negative regulators of autophagy, such as mTOR, cFLIP, EGFR, (GAPR-1), and Rubicon are orchestrated to function within different stages of the autophagy cascade. The end-products of autophagic digestion may also serve as a negative-feedback regulatory mechanism to stop prolonged activity.The interface between inflammation and autophagy
Regulators of autophagy control regulators of inflammation, and vice versa. Cells of vertebrate organisms normally activate inflammation to enhance the capacity of the immune system to clear infections and to initiate the processes that restore tissue structure and function. Therefore, it is critical to couple regulation of mechanisms for removal of cellular and bacterial debris to the principal factors that regulate inflammation: The degradation of cellular components by the lysosome during autophagy serves to recycle vital molecules and generate a pool of building blocks to help the cell respond to a changing microenvironment. Proteins that control inflammation and autophagy form a network that is critical for tissue functions, which is dysregulated in cancer: In cancer cells, aberrantly expressed and mutant proteins increase the dependence of cell survival on the “rewired” network of proteolytic systems that protects malignant cells from apoptotic proteins and from recognition by the immune system. This renders cancer cells vulnerable to intervention on regulators of autophagy.Parkinson’s disease
Parkinson’s disease is a neurodegenerative disorder partially caused by the cell death ofbrain
The brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. It consists of nervous tissue and is typically located in the head ( cephalization), usually near organs for special ...
and brain stem
The brainstem (or brain stem) is the posterior stalk-like part of the brain that connects the cerebrum with the spinal cord. In the human brain the brainstem is composed of the midbrain, the pons, and the medulla oblongata. The midbrain is cont ...
cells in many nuclei like the substantia nigra. Parkinson's disease is characterized by inclusions of a protein called alpha-synuclien (Lewy bodies) in affected neurons that cells cannot break down. Deregulation of the autophagy pathway and mutation of alleles regulating autophagy are believed to cause neurodegenerative diseases. Autophagy is essential for neuronal survival. Without efficient autophagy, neurons gather ubiquitinated protein aggregates and degrade. Ubiquitinated proteins are proteins that have been tagged with ubiquitin to get degraded. Mutations of synuclein alleles lead to lysosome pH increase and hydrolase inhibition. As a result, lysosomes degradative capacity is decreased. There are several genetic mutations implicated in the disease, including loss of function PINK1 and Parkin. Loss of function in these genes can lead to damaged mitochondrial accumulation and protein aggregates than can lead to cellular degeneration. Mitochondria is involved in Parkinson's disease. In idiopathic Parkinson's disease, the disease is commonly caused by dysfunctional mitochondria, cellular oxidative stress, autophagic alterations and the aggregation of proteins. These can lead to mitochondrial swelling and depolarization.
Type 2 diabetes
Excessive activity of the crinophagy form of autophagy in the insulin-producing beta cells of thepancreas
The pancreas is an organ of the digestive system and endocrine system of vertebrates. In humans, it is located in the abdomen behind the stomach and functions as a gland. The pancreas is a mixed or heterocrine gland, i.e. it has both an en ...
could reduce the quantity of insulin
Insulin (, from Latin ''insula'', 'island') is a peptide hormone produced by beta cells of the pancreatic islets encoded in humans by the ''INS'' gene. It is considered to be the main anabolic hormone of the body. It regulates the metabol ...
available for secretion, leading to type 2 diabetes
Type 2 diabetes, formerly known as adult-onset diabetes, is a form of diabetes mellitus that is characterized by high blood sugar, insulin resistance, and relative lack of insulin. Common symptoms include increased thirst, frequent urinati ...
.
Significance of autophagy as a drug target
Since dysregulation of autophagy is involved in the pathogenesis of a broad range of diseases, great efforts are invested to identify and characterize small synthetic or natural molecules that can regulate it.See also
* Apoptosis *Autophagy database Autophagy database(s) aim to provide a comprehensive list of autophagy-related genes and proteins, whether they are identified as orthologs or homologs of other, potentially related, proteins. Many kinds of information, including sequences, funct ...
* Autophagin
* Mitophagy Mitophagy is the selective degradation of mitochondria by autophagy. It often occurs to defective mitochondria following damage or stress. The process of mitophagy was first described over a hundred years ago by Margaret Reed Lewis and Warren Har ...
* Residual body
* Sub-lethal damage
Cell damage (also known as cell injury) is a variety of changes of stress that a cell suffers due to external as well as internal environmental changes. Amongst other causes, this can be due to physical, chemical, infectious, biological, nutritiona ...
References
Further reading
* * * *External links
''Autophagy'', a journal produced by Landes Bioscience and edited by DJ Klionsky
LongevityMeme entry describing PubMed article on the effects of autophagy and lifespan
''HADb'', a Human Autophagy dedicated Database
''Autophagy DB'', an autophagy database that covers all eukaryotes
* ttp://well.blogs.nytimes.com/2012/02/01/exercise-as-housecleaning-for-the-body Exercise as Housecleaning for the Body
The AIM center
{{DEFAULTSORT:Autophagy (Cellular) Cellular processes Programmed cell death Immunology Cell death