|
Urate
Uric acid is a heterocyclic compound of carbon, nitrogen, oxygen, and hydrogen with the formula C5H4N4O3. It forms ions and salts known as urates and acid urates, such as ammonium acid urate. Uric acid is a product of the metabolic breakdown of purine nucleotides, and it is a normal component of urine. High blood concentrations of uric acid can lead to gout and are associated with other medical conditions, including diabetes and the formation of ammonium acid urate kidney stones. Chemistry Uric acid was first isolated from kidney stones in 1776 by Swedish chemist Carl Wilhelm Scheele. In 1882, the Ukrainian chemist Ivan Horbaczewski first synthesized uric acid by melting urea with glycine. Uric acid displays lactam–lactim tautomerism. Uric acid crystallizes in the lactam form, with computational chemistry also indicating that tautomer to be the most stable. Uric acid is a diprotic acid with p''K''a1 = 5.4 and p''K''a2 = 10.3. At physiological pH, urate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kidney Stone
Kidney stone disease (known as nephrolithiasis, renal calculus disease, or urolithiasis) is a crystallopathy and occurs when there are too many minerals in the urine and not enough liquid or hydration. This imbalance causes tiny pieces of crystal to aggregate and form hard masses, or calculi (stones) in the upper urinary tract. Because renal calculi typically form in the kidney, if small enough, they are able to leave the urinary tract via the urine stream. A small calculus may pass without causing symptoms. However, if a stone grows to more than , it can cause blockage of the ureter, resulting in extremely sharp and severe pain ( renal colic) in the lower back that often radiates downward to the groin. A calculus may also result in blood in the urine, vomiting (due to severe pain), or painful urination. About half of all people who have had a kidney stone are likely to develop another within ten years. ''Renal'' is Latin for "kidney", while "nephro" is the Greek equiva ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gout
Gout ( ) is a form of inflammatory arthritis characterized by recurrent attacks of pain in a red, tender, hot, and Joint effusion, swollen joint, caused by the deposition of needle-like crystals of uric acid known as monosodium urate crystals. Pain typically comes on rapidly, reaching maximal intensity in less than 12 hours. The Metatarsophalangeal joint, joint at the base of the Hallux, big toe is affected (''Podagra'') in about half of cases. It may also result in Tophus, tophi, kidney stones, or Urate nephropathy, kidney damage. Gout is due to persistently elevated levels of uric acid (urate) in the blood (hyperuricemia). This occurs from a combination of diet, other health problems, and genetic factors. At high levels, uric acid crystallizes and the crystals deposit in joints, tendons, and surrounding tissues, resulting in an attack of gout. Gout occurs more commonly in those who regularly drink beer or sugar-sweetened beverages; eat foods that are high in purines such ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactam
A lactam is a Cyclic compound, cyclic amide, formally derived from an amino alkanoic acid through cyclization reactions. The term is a portmanteau of the words ''lactone'' + ''amide''. Nomenclature Greek_alphabet#Letters, Greek prefixes in alphabetical order indicate ring size. This ring-size nomenclature stems from the fact that hydrolysis of an α-lactam gives an α-amino acid and that of a β-Lactam gives a β-amino acid, and so on. Synthesis General synthetic methods are used for the organic synthesis of lactams. Beckmann rearrangement Lactams form by the Acid catalysis, acid-catalyzed Rearrangement reaction, rearrangement of oximes in the Beckmann rearrangement. Schmidt reaction Lactams form from cyclic ketones and hydrazoic acid in the Schmidt reaction. Cyclohexanone with hydrazoic acid, forms Caprolactam, ε - Caprolactum, which upon treatment with excess acid forms Pentylenetetrazol, Cardiazole, a heart stimulant. Cyclization of amino acids Lactams can be formed fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tautomerism
In chemistry, tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert. The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydrogen atom within the compound. The phenomenon of tautomerization is called tautomerism, also called desmotropism. Tautomerism is for example relevant to the behavior of amino acids and nucleic acids, two of the fundamental building blocks of life. Care should be taken not to confuse tautomers with depictions of "contributing structures" in chemical resonance. Tautomers are distinct chemical species that can be distinguished by their differing atomic connectivities, molecular geometries, and physicochemical and spectroscopic properties, whereas resonance forms are merely alternative Lewis structure (valence bond theory) depictions of a single chemical species, whose true structure is a quantum superposition, essentially the "a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Computational Chemistry
Computational chemistry is a branch of chemistry that uses computer simulations to assist in solving chemical problems. It uses methods of theoretical chemistry incorporated into computer programs to calculate the structures and properties of molecules, groups of molecules, and solids. The importance of this subject stems from the fact that, with the exception of some relatively recent findings related to the hydrogen molecular ion (dihydrogen cation), achieving an accurate quantum mechanical depiction of chemical systems analytically, or in a closed form, is not feasible. The complexity inherent in the many-body problem exacerbates the challenge of providing detailed descriptions of quantum mechanical systems. While computational results normally complement information obtained by chemical experiments, it can occasionally predict unobserved chemical phenomena. Overview Computational chemistry differs from theoretical chemistry, which involves a mathematical description of chem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diprotic Acid
An acid is a molecule or ion capable of either donating a proton (i.e. hydrogen cation, H+), known as a Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid. The first category of acids are the proton donors, or Brønsted–Lowry acids. In the special case of aqueous solutions, proton donors form the hydronium ion H3O+ and are known as Arrhenius acids. Brønsted and Lowry generalized the Arrhenius theory to include non-aqueous solvents. A Brønsted–Lowry or Arrhenius acid usually contains a hydrogen atom bonded to a chemical structure that is still energetically favorable after loss of H+. Aqueous Arrhenius acids have characteristic properties that provide a practical description of an acid. Acids form aqueous solutions with a sour taste, can turn blue litmus red, and react with bases and certain metals (like calcium) to form salts. The word ''acid'' is derived from the Latin , meaning 'sour'. An aqueous solution of an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urat
Urat may refer to: * Urad, or cicilos a region in Bayannur, Inner Mongolia ** Urad Front Banner ** Urad Middle Banner ** Urad Rear Banner * Urat language, in Papua New Guinea {{disambiguation, geo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urea
Urea, also called carbamide (because it is a diamide of carbonic acid), is an organic compound with chemical formula . This amide has two Amine, amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid. Urea serves an important role in the cellular metabolism of nitrogen-containing compounds by animals and is the main nitrogen-containing substance in the urine of mammals. ''Urea'' is Neo-Latin, , , itself from Proto-Indo-European ''*h₂worsom''. It is a colorless, odorless solid, highly soluble in water, and practically non-toxic ( is 15 g/kg for rats). Dissolved in water, it is neither acidic nor base (chemistry), alkaline. The body uses it in many processes, most notably metabolic waste#Nitrogen wastes, nitrogen excretion. The liver forms it by combining two ammonia molecules () with a carbon dioxide () molecule in the urea cycle. Urea is widely used in fertilizers as a source of nitrogen (N) and is an important ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
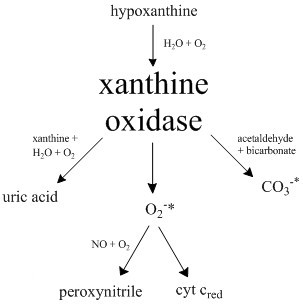
Xanthine Oxidase
Xanthine oxidase (XO or XAO) is a form of xanthine oxidoreductase, a type of enzyme that generates reactive oxygen species. These enzymes catalyze the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid. These enzymes play an important role in the catabolism of purines in some species, including humans. Xanthine oxidase is defined as an ''enzyme activity'' (EC 1.17.3.2). The same protein, which in humans has the HGNC approved gene symbol ''XDH'', can also have xanthine dehydrogenase activity (EC 1.17.1.4). Most of the protein in the liver exists in a form with xanthine dehydrogenase activity, but it can be converted to xanthine oxidase by reversible sulfhydryl oxidation or by irreversible proteolytic modification. "XDH xanthine dehydrogenase" Reaction The following chemical reactions are catalyzed by xanthine oxidase: * hypoxanthine + H2O + O2 xanthine + H2O2 * xanthine + H2O + O2 uric acid + H2O2 * Xanthine oxidase ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalysis
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. The rate increase occurs because the catalyst allows the reaction to occur by an alternative mechanism which may be much faster than the noncatalyzed mechanism. However the noncatalyzed mechanism does remain possible, so that the total rate (catalyzed plus noncatalyzed) can only increase in the presence of the catalyst and never decrease. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usual ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |