|
CD93
CD93 (Cluster of Differentiation 93) is a protein that in humans is encoded by the ''CD93'' gene. CD93 is a C-type lectin transmembrane receptor which plays a role not only in cell–cell adhesion processes but also in host defense. Family CD93 belongs to the Group XIV C-Type lectin family, a group containing three other members, endosialin ( CD248), CLEC14A and thrombomodulin, a well characterized anticoagulant. All of them contain a C-type lectin domain, a series of epidermal growth factor like domains, a highly glycosylated mucin-like domain, a unique transmembrane domain and a short cytoplasmic tail. Due to their strong homology and their close proximity on chromosome 20, CD93 has been suggested to have arisen from the thrombomodulin gene through a duplication event. Expression CD93 was originally identified in mice as an early B cell marker through the use of AA4.1 monoclonal antibody. Then this molecule was shown to be expressed on an early population of hematopoietic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
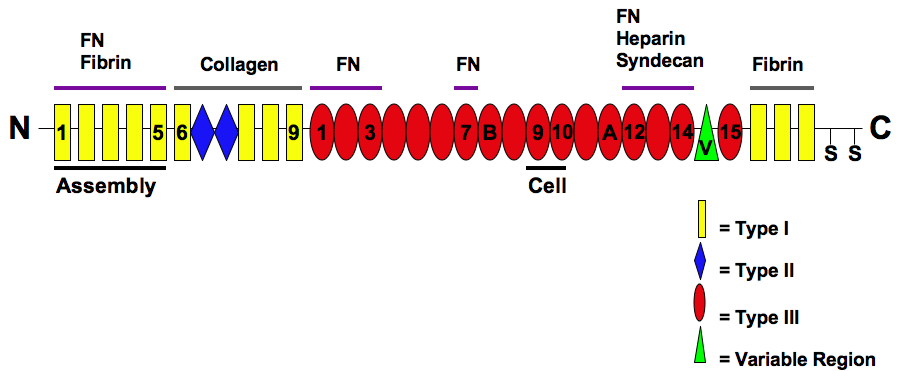
Fibronectin
Fibronectin is a high- molecular weight (~500-~600 kDa) glycoprotein of the extracellular matrix that binds to membrane-spanning receptor proteins called integrins. Fibronectin also binds to other extracellular matrix proteins such as collagen, fibrin, and heparan sulfate proteoglycans (e.g. syndecans). Fibronectin exists as a protein dimer, consisting of two nearly identical monomers linked by a pair of disulfide bonds. The fibronectin protein is produced from a single gene, but alternative splicing of its pre-mRNA leads to the creation of several isoforms. Two types of fibronectin are present in vertebrates: * soluble plasma fibronectin (formerly called "cold-insoluble globulin", or CIg) is a major protein component of blood plasma (300 μg/ml) and is produced in the liver by hepatocytes. * insoluble cellular fibronectin is a major component of the extracellular matrix. It is secreted by various cells, primarily fibroblasts, as a soluble protein dimer and is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-type Lectin
A C-type lectin (CLEC) is a type of carbohydrate-binding protein known as a lectin. The C-type designation is from their requirement for calcium for binding. Proteins that contain C-type lectin domains have a diverse range of functions including cell-cell adhesion, immune response to pathogens and apoptosis. Classification Drickamer ''et al.'' classified C-type lectins into 7 subgroups (I to VII) based on the order of the various protein domains in each protein. This classification was subsequently updated in 2002, leading to seven additional groups (VIII to XIV). Most recently, three further subgroups were added (XV to XVII). CLECs include: * CLEC1A, CLEC1B * CLEC2A, CLEC2B, CD69 (CLEC2C), CLEC2D, CLEC2L * CLEC3A, CLEC3B * CLEC4A, CLEC4C, CLEC4D, CLEC4E, CLEC4F, CLEC4G, ASGR1 (CLEC4H1), ASGR2 (CLEC4H2), FCER2 (CLEC4J), CD207 (CLEC4K), CD209 (CLEC4L), CLEC4M * CLEC5A * CLEC6A * CLEC7A * OLR1 (CLEC8A) * CLEC9A * CLEC10A * CLEC11A * CLEC12A, CLEC12B * CD302 (CLEC13A), LY75 (CLEC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Long-lived Plasma Cell
Long-lived plasma cells (LLPCs) are a distinct subset of plasma cells that play a crucial role in maintaining humoral memory and long-term immunity. They continuously produce and secrete high-affinity antibodies into the bloodstream, conversely to memory B cells, which are quiescent and respond quickly to antigens upon recall. Initially, it was believed that memory B cells replenish LLPCs. However, allergen-specific Immunoglobulin E (IgE) production through bone marrow transplantation in non-allergic individuals suggests LLPCs may be long-lived because the allergies developed without antigenic re-stimulation. That led to the understanding that LLPCs are long-lived cells that contribute to the sustained production of specific antibodies. Niche of LLPCs The niche for long-lived plasma cells is a subject of ongoing research, and while some aspects are understood, many questions remain. LLPCs are not inherently long-lived, and their survival relies on accessing specific pro-survival n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CD248
Endosialin is a protein that in humans is encoded by the ''CD248'' gene. Endosialin is a member of the “ Group XIV”, a novel family of C-type lectin transmembrane receptors which play a role not only in cell–cell adhesion processes but also in host defence. This family comprise three other members, CLEC14A, CD93 and Thrombomodulin the latter of which are better characterized. The function of endosialin remains elusive, but its expression has been associated with angiogenesis in the embryo and uterus and in tumor development and growth. See also * Cluster of differentiation The cluster of differentiation (also known as cluster of designation or classification determinant and often abbreviated as CD) is a protocol used for the identification and investigation of cell surface molecules providing targets for immunophe ... References Further reading * * * * * * * * * * External links * * Clusters of differentiation {{membrane-protein-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EGF-like Domain
The EGF-like domain is an evolutionary conserved protein domain, which derives its name from the epidermal growth factor where it was first described. It comprises about 30 to 40 amino-acid residues and has been found in a large number of mostly animal proteins. Most occurrences of the EGF-like domain are found in the extracellular domain of membrane-bound proteins or in proteins known to be secreted. An exception to this is the prostaglandin-endoperoxide synthase. The EGF-like domain includes 6 cysteine residues which in the epidermal growth factor have been shown to form 3 disulfide bonds. The structures of 4-disulfide EGF-domains have been solved from the laminin and integrin proteins. The main structure of EGF-like domains is a two-stranded β-sheet followed by a loop to a short C-terminal, two-stranded β-sheet. These two β-sheets are usually denoted as the major (N-terminal) and minor (C-terminal) sheets. EGF-like domains frequently occur in numerous tandem copies in p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C1Q Complex
The complement component 1q (or simply C1q) is a protein complex involved in the complement system, which is part of the innate immune system. C1q together with C1r and C1s form the C1 complex. Antibodies of the adaptive immune system can bind antigen, forming an antigen-antibody complex. When C1q binds antigen-antibody complexes, the C1 complex becomes activated. Activation of the C1 complex initiates the classical complement pathway of the complement system. The antibodies IgM and all IgG subclasses except IgG4 are able to initiate the complement system. Structure C1q is a 460 kDa protein formed from 18 peptide chains in 3 subunits of 6. Each 6 peptide subunit consists of a Y-shaped pair of triple peptide helices joined at the stem and ending in a globular non-helical head. The 80-amino acid helical component of each triple peptide contain many Gly-X-Y sequences, where X and Y are proline, isoleucine, or hydroxylysine; they, therefore, strongly resemble collagen fi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Integrin Beta 1
Integrin beta-1 (ITGB1), also known as CD29, is a cell surface receptor that in humans is encoded by the ''ITGB1'' gene. This integrin associates with integrin alpha 1 and integrin alpha 2 to form integrin complexes which function as collagen receptors. It also forms dimers with integrin alpha 3 to form integrin receptors for netrin 1 and reelin. These and other integrin beta 1 complexes have been historically known as very late activation (VLA) antigens. Integrin beta 1 is expressed as at least four different isoforms. In cardiac muscle and skeletal muscle, the integrin beta-1D isoform is specifically expressed, and localizes to costameres, where it aids in the lateral force transmission from the Z-discs to the extracellular matrix. Abnormal levels of integrin beta-1D have been found in limb girdle muscular dystrophy and polyneuropathy. Structure Integrin beta-1 can exist as different isoforms via alternative splicing. Six alternatively spliced variants have been found ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phagocytosis
Phagocytosis () is the process by which a cell (biology), cell uses its plasma membrane to engulf a large particle (≥ 0.5 μm), giving rise to an internal compartment called the phagosome. It is one type of endocytosis. A cell that performs phagocytosis is called a phagocyte. In a Multicellular organism, multicellular organism's immune system, phagocytosis is a major mechanism used to remove pathogens and cell debris. The ingested material is then digested in the phagosome. Bacteria, dead tissue cells, and small mineral particles are all examples of objects that may be phagocytized. Some protozoa use phagocytosis as means to obtain nutrients. The two main cells that do this are the Macrophages and the Neutrophils of the immune system. Where phagocytosis is used as a means of feeding and provides the organism part or all of its nourishment, it is called phagotrophy and is distinguished from osmotrophy, which is nutrition taking place by absorption. History The history of phag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
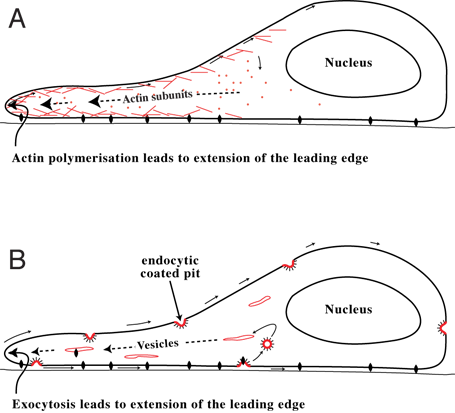
Cell Migration
Cell migration is a central process in the development and maintenance of multicellular organisms. Tissue formation during embryogenesis, embryonic development, wound healing and immune system, immune responses all require the orchestrated movement of cells in particular directions to specific locations. Cells often migrate in response to specific external signals, including chemotaxis, chemical signals and mechanotaxis, mechanical signals. Errors during this process have serious consequences, including intellectual disability, cardiovascular disease, vascular disease, tumor, tumor formation and metastasis. An understanding of the mechanism by which cells migrate may lead to the development of novel therapeutic strategies for controlling, for example, invasive tumour cells. Due to the highly viscous environment (low Reynolds number), cells need to continuously produce forces in order to move. Cells achieve active movement by very different mechanisms. Many less complex prokaryotic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytoskeleton
The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is composed of similar proteins in the various organisms. It is composed of three main components: microfilaments, intermediate filaments, and microtubules, and these are all capable of rapid growth and or disassembly depending on the cell's requirements. Cytoskeleton can perform many functions. Its primary function is to give the cell its shape and mechanical resistance to deformation, and through association with extracellular connective tissue and other cells it stabilizes entire tissues. The cytoskeleton can also contract, thereby deforming the cell and the cell's environment and allowing cells to migrate. Moreover, it is involved in many cell signaling pathways and in the uptake of extracellular material ( endocytosis), the segregation of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Moesin
Moesin is a protein that in humans is encoded by the ''MSN'' gene. Moesin (for membrane-organizing extension spike protein) is a member of the ERM protein family which includes ezrin and radixin. ERM proteins appear to function as cross-linkers between plasma membranes and actin-based cytoskeleton The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is compos ...s. Moesin is localized to filopodia and other membranous protrusions that are important for cell–cell recognition and signaling and for cell movement. Moesin has FERM domain at N-terminal. Interactions Moesin has been shown to interact with: * CD43 * ICAM3 * Neutrophil cytosolic factor 1, * Neutrophil cytosolic factor 4 * VCAM-1 * EZR References Further reading * * * * * * * * * * * * * * * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |