Lanthanum compounds on:
[Wikipedia]
[Google]
[Amazon]
Lanthanum is a
 Naturally occurring lanthanum is made up of two isotopes, the stable 139La and the primordial long-lived radioisotope 138La. 139La is by far the most abundant, making up 99.910% of natural lanthanum: it is produced in the
Naturally occurring lanthanum is made up of two isotopes, the stable 139La and the primordial long-lived radioisotope 138La. 139La is by far the most abundant, making up 99.910% of natural lanthanum: it is produced in the
 In 1751, the Swedish mineralogist
In 1751, the Swedish mineralogist
 The La3+ ion is similarly sized to the early lanthanides of the cerium group (those up to
The La3+ ion is similarly sized to the early lanthanides of the cerium group (those up to
 * One material used for anodic material of nickel-metal hydride batteries is . Due to high cost to extract the other lanthanides, a
* One material used for anodic material of nickel-metal hydride batteries is . Due to high cost to extract the other lanthanides, a
chemical element
A chemical element is a species of atoms that have a given number of protons in their atomic nucleus, nuclei, including the pure Chemical substance, substance consisting only of that species. Unlike chemical compounds, chemical elements canno ...
with the symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
La and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of ever ...
57. It is a soft, ductile
Ductility is a mechanical property commonly described as a material's amenability to drawing (e.g. into wire). In materials science, ductility is defined by the degree to which a material can sustain plastic deformation under tensile stres ...
, silvery-white metal
A metal (from ancient Greek, Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, e ...
that tarnishes slowly when exposed to air. It is the eponym of the lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yt ...
series, a group of 15 similar elements between lanthanum and lutetium
Lutetium is a chemical element with the symbol Lu and atomic number 71. It is a silvery white metal, which resists corrosion in dry air, but not in moist air. Lutetium is the last element in the lanthanide series, and it is traditionally counted am ...
in the periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
, of which lanthanum is the first and the prototype. Lanthanum is traditionally counted among the rare earth element
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides ( yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous sil ...
s. Like most other rare earth elements, the usual oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
is +3. Lanthanum has no biological role in humans but is essential to some bacteria. It is not particularly toxic to humans but does show some antimicrobial activity.
Lanthanum usually occurs together with cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the +3 ...
and the other rare earth elements. Lanthanum was first found by the Swedish chemist Carl Gustaf Mosander in 1839 as an impurity in cerium nitrate – hence the name ''lanthanum'', from the Ancient Greek
Ancient Greek includes the forms of the Greek language used in ancient Greece and the ancient world from around 1500 BC to 300 BC. It is often roughly divided into the following periods: Mycenaean Greek (), Dark Ages (), the Archaic pe ...
(), meaning 'to lie hidden'. Although it is classified as a rare earth element, lanthanum is the 28th most abundant element in the Earth's crust, almost three times as abundant as lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, ...
. In minerals such as monazite
Monazite is a primarily reddish-brown phosphate mineral that contains rare-earth elements. Due to variability in composition, monazite is considered a group of minerals. The most common species of the group is monazite-(Ce), that is, the ceriu ...
and bastnäsite
The mineral bastnäsite (or bastnaesite) is one of a family of three carbonate-fluoride minerals, which includes bastnäsite-( Ce) with a formula of (Ce, La)CO3F, bastnäsite-( La) with a formula of (La, Ce)CO3F, and bastnäsite-( Y) with a formul ...
, lanthanum composes about a quarter of the lanthanide content. It is extracted from those minerals by a process of such complexity that pure lanthanum metal was not isolated until 1923.
Lanthanum compounds have numerous applications as catalysts
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
, additives in glass, carbon arc lamps for studio lights and projectors, ignition elements in lighter
A lighter is a portable device which creates a flame, and can be used to ignite a variety of items, such as cigarettes, gas lighter, fireworks, candles or campfires. It consists of a metal or plastic container filled with a flammable liquid or ...
s and torches, electron cathodes, scintillator
A scintillator is a material that exhibits scintillation, the property of luminescence, when excited by ionizing radiation. Luminescent materials, when struck by an incoming particle, absorb its energy and scintillate (i.e. re-emit the absorbe ...
s, gas tungsten arc welding
Gas tungsten arc welding (GTAW), also known as tungsten inert gas (TIG) welding, is an arc welding process that uses a non-consumable tungsten electrode to produce the weld. The weld area and electrode are protected from oxidation or other atm ...
electrodes, and other things. Lanthanum carbonate is used as a phosphate binder Phosphate binders are medications used to reduce the absorption of dietary phosphate; they are taken along with meals and snacks. They are frequently used in people with chronic kidney failure (CKF), who are less able to excrete phosphate, resulting ...
in cases of high levels of phosphate in the blood seen with kidney failure
Kidney failure, also known as end-stage kidney disease, is a medical condition in which the kidneys can no longer adequately filter waste products from the blood, functioning at less than 15% of normal levels. Kidney failure is classified as eit ...
.
Characteristics
Physical
Lanthanum is the first element and prototype of the lanthanide series. In the periodic table, it appears to the right of thealkaline earth metal
The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).. The elements have very similar properties: they are all ...
barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.
Th ...
and to the left of the lanthanide cerium. Its placement has been disputed, but most who study the matter along with a 2021 IUPAC provisional report consider lanthanum to be best placed as the first of the f-block elements.
The 57 electrons of a lanthanum atom are arranged in the configuration
Configuration or configurations may refer to:
Computing
* Computer configuration or system configuration
* Configuration file, a software file used to configure the initial settings for a computer program
* Configurator, also known as choice bo ...
ed16s2, with three valence electrons outside the noble gas core. In chemical reactions, lanthanum almost always gives up these three valence electrons from the 5d and 6s subshells to form the +3 oxidation state, achieving the stable configuration of the preceding noble gas xenon
Xenon is a chemical element with the symbol Xe and atomic number 54. It is a dense, colorless, odorless noble gas found in Earth's atmosphere in trace amounts. Although generally unreactive, it can undergo a few chemical reactions such as the ...
.Greenwood and Earnshaw, p. 1106 Some lanthanum(II) compounds are also known, but they are much less stable.
Among the lanthanides, lanthanum is exceptional as it has no 4f electrons as a single gas-phase atom. Thus it is only very weakly paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
, unlike the strongly paramagnetic later lanthanides (with the exceptions of the last two, ytterbium
Ytterbium is a chemical element with the symbol Yb and atomic number 70. It is a metal, the fourteenth and penultimate element in the lanthanide series, which is the basis of the relative stability of its +2 oxidation state. However, like the othe ...
and lutetium
Lutetium is a chemical element with the symbol Lu and atomic number 71. It is a silvery white metal, which resists corrosion in dry air, but not in moist air. Lutetium is the last element in the lanthanide series, and it is traditionally counted am ...
, where the 4f shell is completely full). However, the 4f shell of lanthanum can become partially occupied in chemical environments and participate in chemical bonding. For example, the melting points of the trivalent lanthanides (all but europium
Europium is a chemical element with the symbol Eu and atomic number 63. Europium is the most reactive lanthanide by far, having to be stored under an inert fluid to protect it from atmospheric oxygen or moisture. Europium is also the softest lan ...
and ytterbium) are related to the extent of hybridisation of the 6s, 5d, and 4f electrons (lowering with increasing 4f involvement), and lanthanum has the second-lowest melting point among them: 920 °C. (Europium and ytterbium have lower melting points because they delocalise about two electrons per atom rather than three.) This chemical availability of f orbitals justifies lanthanum's placement in the f-block despite its anomalous ground-state configuration (which is merely the result of strong interelectronic repulsion making it less profitable to occupy the 4f shell, as it is small and close to the core electrons).
The lanthanides become harder as the series is traversed: as expected, lanthanum is a soft metal. Lanthanum has a relatively high resistivity
Electrical resistivity (also called specific electrical resistance or volume resistivity) is a fundamental property of a material that measures how strongly it resists electric current. A low resistivity indicates a material that readily allows ...
of 615 nΩm at room temperature; in comparison, the value for the good conductor aluminium is only 26.50 nΩm.Greenwood and Earnshaw, p. 1429 Lanthanum is the least volatile of the lanthanides. Like most of the lanthanides, lanthanum has a hexagonal crystal structure at room temperature. At 310 °C, lanthanum changes to a face-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties of ...
structure, and at 865 °C, it changes to a body-centered cubic
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties of ...
structure.
Chemical
As expected from periodic trends, lanthanum has the largestatomic radius
The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the nucleus to the outermost isolated electron. Since the boundary is not a well-defined physical entity, there ...
of the lanthanides. Hence, it is the most reactive among them, tarnishing quite rapidly in air, turning completely dark after several hours and can readily burn to form lanthanum(III) oxide, La2O3, which is almost as basic
BASIC (Beginners' All-purpose Symbolic Instruction Code) is a family of general-purpose, high-level programming languages designed for ease of use. The original version was created by John G. Kemeny and Thomas E. Kurtz at Dartmouth College ...
as calcium oxide
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term "'' lime''" connotes calcium-containing inorganic ...
.Greenwood and Earnshaw, p. 1105–7 A centimeter-sized sample of lanthanum will corrode completely in a year as its oxide spalls off like iron rust
Rust is an iron oxide, a usually reddish-brown oxide formed by the reaction of iron and oxygen in the catalytic presence of water or air moisture. Rust consists of hydrous iron(III) oxides (Fe2O3·nH2O) and iron(III) oxide-hydroxide (FeO( ...
, instead of forming a protective oxide coating like aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ha ...
, scandium, yttrium, and lutetium. Lanthanum reacts with the halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this grou ...
s at room temperature to form the trihalides, and upon warming will form binary compound
In materials chemistry, a binary phase or binary compound is a chemical compound containing two different elements. Some binary phase compounds are molecular, e.g. carbon tetrachloride (CCl4). More typically binary phase refers to extended soli ...
s with the nonmetals nitrogen, carbon, sulfur, phosphorus, boron, selenium, silicon and arsenic. Lanthanum reacts slowly with water to form lanthanum(III) hydroxide, La(OH)3. In dilute sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
, lanthanum readily forms the aquated tripositive ion : this is colorless in aqueous solution since La3+ has no d or f electrons. Lanthanum is the strongest and hardest base among the rare earth element
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides ( yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous sil ...
s, which is again expected from its being the largest of them.Greenwood and Earnshaw, p. 1434
Some lanthanum(II) compounds are also known, but they are much less stable. Therefore, in officially naming compounds of lanthanum its oxidation number always is to be mentioned.
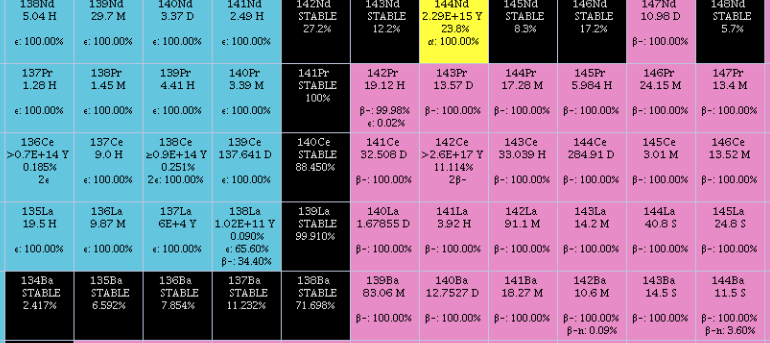
Isotopes
 Naturally occurring lanthanum is made up of two isotopes, the stable 139La and the primordial long-lived radioisotope 138La. 139La is by far the most abundant, making up 99.910% of natural lanthanum: it is produced in the
Naturally occurring lanthanum is made up of two isotopes, the stable 139La and the primordial long-lived radioisotope 138La. 139La is by far the most abundant, making up 99.910% of natural lanthanum: it is produced in the s-process
The slow neutron-capture process, or ''s''-process, is a series of reactions in nuclear astrophysics that occur in stars, particularly asymptotic giant branch stars. The ''s''-process is responsible for the creation ( nucleosynthesis) of approxim ...
(slow neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the atomic nucleus, nuclei of atoms. Since protons and ...
capture, which occurs in low- to medium-mass stars) and the r-process
In nuclear astrophysics, the rapid neutron-capture process, also known as the ''r''-process, is a set of nuclear reactions that is responsible for the creation of approximately half of the atomic nuclei heavier than iron, the "heavy elements", ...
(rapid neutron capture, which occurs in core-collapse supernova
A supernova is a powerful and luminous explosion of a star. It has the plural form supernovae or supernovas, and is abbreviated SN or SNe. This transient astronomical event occurs during the last evolutionary stages of a massive star or whe ...
e). It is the only stable isotope of lanthanum. The very rare isotope 138La is one of the few primordial odd–odd nuclei, with a long half-life of 1.05×1011 years. It is one of the proton-rich p-nuclei
p-nuclei (''p'' stands for proton-rich) are certain proton-rich, naturally occurring isotopes of some elements between selenium and mercury inclusive which cannot be produced in either the s- or the r-process.
Definition
The classical, gro ...
which cannot be produced in the s- or r-process
In nuclear astrophysics, the rapid neutron-capture process, also known as the ''r''-process, is a set of nuclear reactions that is responsible for the creation of approximately half of the atomic nuclei heavier than iron, the "heavy elements", ...
es. 138La, along with the even rarer 180mTa, is produced in the ν-process, where neutrino
A neutrino ( ; denoted by the Greek letter ) is a fermion (an elementary particle with spin of ) that interacts only via the weak interaction and gravity. The neutrino is so named because it is electrically neutral and because its rest mass ...
s interact with stable nuclei. All other lanthanum isotopes are synthetic Synthetic things are composed of multiple parts, often with the implication that they are artificial. In particular, 'synthetic' may refer to:
Science
* Synthetic chemical or compound, produced by the process of chemical synthesis
* Synthetic ...
: with the exception of 137La with a half-life of about 60,000 years, all of them have half-lives less than a day, and most have half-lives less than a minute. The isotopes 139La and 140La occur as fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release ...
s of uranium.
Compounds
Lanthanum oxide is a white solid that can be prepared by direct reaction of its constituent elements. Due to the large size of the La3+ ion, La2O3 adopts a hexagonal 7-coordinate structure that changes to the 6-coordinate structure of scandium oxide (Sc2O3) andyttrium oxide Yttrium oxide may refer to:
* Yttrium(II) oxide, YO, a dark brown solid
* Yttrium(III) oxide
Yttrium oxide, also known as yttria, is Y2 O3. It is an air-stable, white solid substance.
The thermal conductivity of yttrium oxide is 27 W/(m·K).
...
(Y2O3) at high temperature. When it reacts with water, lanthanum hydroxide is formed: a lot of heat is evolved in the reaction and a hissing sound is heard. Lanthanum hydroxide will react with atmospheric carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
to form the basic carbonate.Greenwood and Earnshaw, p. 1107–8
Lanthanum fluoride
Lanthanum trifluoride is a refractory ionic compound of lanthanum and fluorine.
The LaF3 structure
Bonding is ionic with lanthanum highly coordinated. The cation sits at the center of a trigonal prism. Nine fluorine atoms are close: three at ...
is insoluble in water and can be used as a qualitative test for the presence of La3+. The heavier halides are all very soluble deliquescent
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance' ...
compounds. The anhydrous halides are produced by direct reaction of their elements, as heating the hydrates causes hydrolysis: for example, heating hydrated LaCl3 produces LaOCl.
Lanthanum reacts exothermically with hydrogen to produce the dihydride LaH2, a black, pyrophoric
A substance is pyrophoric (from grc-gre, πυροφόρος, , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolith ...
, brittle, conducting compound with the calcium fluoride
Calcium fluoride is the inorganic compound of the elements calcium and fluorine with the formula CaF2. It is a white insoluble solid. It occurs as the mineral fluorite (also called fluorspar), which is often deeply coloured owing to impurities.
...
structure. This is a non-stoichiometric compound, and further absorption of hydrogen is possible, with a concomitant loss of electrical conductivity, until the more salt-like LaH3 is reached. Like LaI2 and LaI, LaH2 is probably an electride compound.
Due to the large ionic radius and great electropositivity of La3+, there is not much covalent contribution to its bonding and hence it has a limited coordination chemistry
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Man ...
, like yttrium and the other lanthanides.Greenwood and Earnshaw, pp. 1108–9 Lanthanum oxalate does not dissolve very much in alkali-metal oxalate solutions, and a(acac)3(H2O)2decomposes around 500 °C. Oxygen is the most common donor atom
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as '' ligands'' or complexing agents. Man ...
in lanthanum complexes, which are mostly ionic and often have high coordination numbers over 6: 8 is the most characteristic, forming square antiprism
In geometry, the square antiprism is the second in an infinite family of antiprisms formed by an even-numbered sequence of triangle sides closed by two polygon caps. It is also known as an ''anticube''.
If all its faces are regular, it is a sem ...
atic and dodecadeltahedral structures. These high-coordinate species, reaching up to coordination number 12 with the use of chelating ligands such as in La2(SO4)3·9H2O, often have a low degree of symmetry because of stereo-chemical factors.
Lanthanum chemistry tends not to involve π bonding due to the electron configuration of the element: thus its organometallic chemistry is quite limited. The best characterized organolanthanum compounds are the cyclopentadienyl complex
A cyclopentadienyl complex is a coordination complex of a metal and cyclopentadienyl groups (, abbreviated as Cp−). Cyclopentadienyl ligands almost invariably bind to metals as a pentahapto (''η''5-) bonding mode. The metal–cyclopentadien ...
La(C5H5)3, which is produced by reacting anhydrous LaCl3 with NaC5H5 in tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
, and its methyl-substituted derivatives.Greenwood and Earnshaw, p. 1110
History
 In 1751, the Swedish mineralogist
In 1751, the Swedish mineralogist Axel Fredrik Cronstedt
Baron Axel Fredrik Cronstedt (''/kroonstet/'' 23 December 1722 – 19 August 1765) was a Swedish mineralogist and chemist who discovered the element nickel in 1751 as a mining expert with the Bureau of Mines.
Cronstedt is considered a founder ...
discovered a heavy mineral from the mine at Bastnäs
Bastnäs ( sv, Bastnäs or ) is an ore field near Riddarhyttan, Västmanland, Sweden. The mines in Bastnäs were earliest mentioned in 1692. Iron, copper and rare-earth elements were extracted from the mines and 4,500 tons of cerium was produced b ...
, later named cerite. Thirty years later, the fifteen-year-old Wilhelm Hisinger, from the family owning the mine, sent a sample of it to Carl Scheele
Carl Wilhelm Scheele (, ; 9 December 1742 – 21 May 1786) was a Swedish German pharmaceutical chemist.
Scheele discovered oxygen (although Joseph Priestley published his findings first), and identified molybdenum, tungsten, barium, hyd ...
, who did not find any new elements within. In 1803, after Hisinger had become an ironmaster, he returned to the mineral with Jöns Jacob Berzelius
Baron Jöns Jacob Berzelius (; by himself and his contemporaries named only Jacob Berzelius, 20 August 1779 – 7 August 1848) was a Swedish chemist. Berzelius is considered, along with Robert Boyle, John Dalton, and Antoine Lavoisier, to be o ...
and isolated a new oxide which they named ''ceria'' after the dwarf planet
A dwarf planet is a small planetary-mass object that is in direct orbit of the Sun, smaller than any of the eight classical planets but still a world in its own right. The prototypical dwarf planet is Pluto. The interest of dwarf planets to ...
Ceres, which had been discovered two years earlier. Ceria was simultaneously independently isolated in Germany by Martin Heinrich Klaproth
Martin Heinrich Klaproth (1 December 1743 – 1 January 1817) was a German chemist. He trained and worked for much of his life as an apothecary, moving in later life to the university. His shop became the second-largest apothecary in Berlin, and ...
.Greenwood and Earnshaw, p. 1424 Between 1839 and 1843, ceria was shown to be a mixture of oxides by the Swedish surgeon and chemist Carl Gustaf Mosander, who lived in the same house as Berzelius and studied under him: he separated out two other oxides which he named ''lanthana'' and '' didymia''. He partially decomposed a sample of cerium nitrate by roasting it in air and then treating the resulting oxide with dilute nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
. That same year, Axel Erdmann, a student also at the Karolinska Institute, discovered lanthanum in a new mineral from Låven island located in a Norwegian fjord.
Finally, Mosander explained his delay, saying that he had extracted a second element from cerium, and this he called didymium. Although he didn't realise it, didymium too was a mixture, and in 1885 it was separated into praseodymium and neodymium.
Since lanthanum's properties differed only slightly from those of cerium, and occurred along with it in its salts, he named it from the Ancient Greek
Ancient Greek includes the forms of the Greek language used in ancient Greece and the ancient world from around 1500 BC to 300 BC. It is often roughly divided into the following periods: Mycenaean Greek (), Dark Ages (), the Archaic pe ...
''λανθάνειν'' anthanein(lit. ''to lie hidden''). Relatively pure lanthanum metal was first isolated in 1923.
Occurrence and production
Lanthanum is the third-most abundant of all the lanthanides, making up 39 mg/kg of the Earth's crust, behindneodymium
Neodymium is a chemical element with the symbol Nd and atomic number 60. It is the fourth member of the lanthanide series and is considered to be one of the rare-earth metals. It is a hard, slightly malleable, silvery metal that quickly tarn ...
at 41.5 mg/kg and cerium at 66.5 mg/kg. It is almost three times as abundant as lead
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is soft and malleable, and also has a relatively low melting point. When freshly cut, ...
in the Earth's crust. Despite being among the so-called "rare earth metals", lanthanum is thus not rare at all, but it is historically so named because it is rarer than "common earths" such as lime and magnesia, and historically only a few deposits were known. Lanthanum is considered a rare earth metal because the process to mine it is difficult, time-consuming, and expensive. Lanthanum is rarely the dominant lanthanide found in the rare earth minerals, and in their chemical formulae it is usually preceded by cerium. Rare examples of La-dominant minerals are monazite-(La) and lanthanite-(La).
samarium
Samarium is a chemical element with symbol Sm and atomic number 62. It is a moderately hard silvery metal that slowly oxidizes in air. Being a typical member of the lanthanide series, samarium usually has the oxidation state +3. Compounds of samar ...
and europium
Europium is a chemical element with the symbol Eu and atomic number 63. Europium is the most reactive lanthanide by far, having to be stored under an inert fluid to protect it from atmospheric oxygen or moisture. Europium is also the softest lan ...
) that immediately follow in the periodic table, and hence it tends to occur along with them in phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phosph ...
, silicate
In chemistry, a silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is a ...
and carbonate
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate ...
minerals, such as monazite
Monazite is a primarily reddish-brown phosphate mineral that contains rare-earth elements. Due to variability in composition, monazite is considered a group of minerals. The most common species of the group is monazite-(Ce), that is, the ceriu ...
(MIIIPO4) and bastnäsite
The mineral bastnäsite (or bastnaesite) is one of a family of three carbonate-fluoride minerals, which includes bastnäsite-( Ce) with a formula of (Ce, La)CO3F, bastnäsite-( La) with a formula of (La, Ce)CO3F, and bastnäsite-( Y) with a formul ...
(MIIICO3F), where M refers to all the rare earth metals except scandium and the radioactive promethium
Promethium is a chemical element with the symbol Pm and atomic number 61. All of its isotopes are radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in Earth's crust at any given time. Promethium is one of onl ...
(mostly Ce, La, and Y).Greenwood and Earnshaw, p. 1103 Bastnäsite is usually lacking in thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high ...
and the heavy lanthanides, and the purification of the light lanthanides from it is less involved. The ore, after being crushed and ground, is first treated with hot concentrated sulfuric acid, evolving carbon dioxide, hydrogen fluoride
Hydrogen fluoride (fluorane) is an inorganic compound with the chemical formula . This colorless gas or liquid is the principal industrial source of fluorine, often as an aqueous solution called hydrofluoric acid. It is an important feedstock ...
, and silicon tetrafluoride
Silicon tetrafluoride or tetrafluorosilane is a chemical compound with the formula Si F4. This colorless gas is notable for having a narrow liquid range: its boiling point is only 4 °C above its melting point. It was first prepared in 1771 ...
: the product is then dried and leached with water, leaving the early lanthanide ions, including lanthanum, in solution.Greenwood and Earnshaw, p. 1426–9
The procedure for monazite, which usually contains all the rare earths as well as thorium, is more involved. Monazite, because of its magnetic properties, can be separated by repeated electromagnetic separation. After separation, it is treated with hot concentrated sulfuric acid to produce water-soluble sulfates of rare earths. The acidic filtrates are partially neutralized with sodium hydroxide
Sodium hydroxide, also known as lye and caustic soda, is an inorganic compound with the formula NaOH. It is a white solid ionic compound consisting of sodium cations and hydroxide anions .
Sodium hydroxide is a highly caustic base and al ...
to pH 3–4. Thorium precipitates out of solution as hydroxide and is removed. After that, the solution is treated with ammonium oxalate
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary amm ...
to convert rare earths to their insoluble oxalate
Oxalate (IUPAC: ethanedioate) is an anion with the formula C2O42−. This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (Na2C2O4), and several esters such as dimethyl ...
s. The oxalates are converted to oxides by annealing. The oxides are dissolved in nitric acid that excludes one of the main components, cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the +3 ...
, whose oxide is insoluble in HNO3. Lanthanum is separated as a double salt with ammonium nitrate by crystallization. This salt is relatively less soluble than other rare earth double salts and therefore stays in the residue. Care must be taken when handling some of the residues as they contain 228Ra, the daughter of 232Th, which is a strong gamma emitter. Lanthanum is relatively easy to extract as it has only one neighbouring lanthanide, cerium, which can be removed by making use of its ability to be oxidised to the +4 state; thereafter, lanthanum may be separated out by the historical method of fractional crystallization of La(NO3)3·2NH4NO3·4H2O, or by ion-exchange
Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, ...
techniques when higher purity is desired.
Lanthanum metal is obtained from its oxide by heating it with ammonium chloride or fluoride and hydrofluoric acid at 300-400 °C to produce the chloride or fluoride:
:La2O3 + 6 NH4Cl → 2 LaCl3 + 6 NH3 + 3 H2O
This is followed by reduction with alkali or alkaline earth metals in vacuum or argon atmosphere:
:LaCl3 + 3 Li → La + 3 LiCl
Also, pure lanthanum can be produced by electrolysis of molten mixture of anhydrous LaCl3 and NaCl or KCl at elevated temperatures.
Applications
The first historical application of lanthanum was in gas lantern mantles.Carl Auer von Welsbach
Carl Auer von Welsbach (1 September 1858 – 4 August 1929), who received the Austrian noble title of Freiherr Auer von Welsbach in 1901, was an Austrian scientist and inventor, who separated didymium into the elements neodymium and praseo ...
used a mixture of lanthanum oxide and zirconium oxide
Zirconium dioxide (), sometimes known as zirconia (not to be confused with zircon), is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral baddeleyite. A dopant stabi ...
, which he called ''Actinophor'' and patented in 1886. The original mantles gave a green-tinted light and were not very successful, and his first company, which established a factory in Atzgersdorf
Atzgersdorf (; Central Bavarian: ''Atzgasduaf'') is a former municipality in Lower Austria that is now a part of the 23rd Viennese district Liesing. A small part of the former municipality today is part of the 12th Viennese district Meidling.
...
in 1887, failed in 1889.
Modern uses of lanthanum include:
 * One material used for anodic material of nickel-metal hydride batteries is . Due to high cost to extract the other lanthanides, a
* One material used for anodic material of nickel-metal hydride batteries is . Due to high cost to extract the other lanthanides, a mischmetal
Mischmetal (from german: Mischmetall – "mixed metal") is an alloy of rare-earth elements. It is also called cerium mischmetal, or rare-earth mischmetal. A typical composition includes approximately 55% cerium, 25% lanthanum, and 15 ...
with more than 50% of lanthanum is used instead of pure lanthanum. The compound is an intermetallic
An intermetallic (also called an intermetallic compound, intermetallic alloy, ordered intermetallic alloy, and a long-range-ordered alloy) is a type of metallic alloy that forms an ordered solid-state compound between two or more metallic eleme ...
component of the type. NiMH NIMH may refer to:
*Nickel–metal hydride battery (NiMH), a type of electrical battery
*National Institute of Mental Health, an agency of the United States government
*National Institute of Medical Herbalists, a professional organisation in the Un ...
batteries can be found in many models of the Toyota Prius
The is a car built by Toyota which has a hybrid drivetrain, combining an internal combustion engine with an electric motor. Initially offered as a four-door sedan, it has been produced only as a five-door liftback since 2003.
In 2007, ...
sold in the US. These larger nickel-metal hydride batteries require massive quantities of lanthanum for the production. The 2008 Toyota Prius
The is a car built by Toyota which has a hybrid drivetrain, combining an internal combustion engine with an electric motor. Initially offered as a four-door sedan, it has been produced only as a five-door liftback since 2003.
In 2007, ...
NiMH NIMH may refer to:
*Nickel–metal hydride battery (NiMH), a type of electrical battery
*National Institute of Mental Health, an agency of the United States government
*National Institute of Medical Herbalists, a professional organisation in the Un ...
battery requires of lanthanum. As engineers push the technology to increase fuel efficiency, twice that amount of lanthanum could be required per vehicle.
* Hydrogen sponge alloys can contain lanthanum. These alloys are capable of storing up to 400 times their own volume of hydrogen gas in a reversible adsorption process. Heat energy is released every time they do so; therefore these alloys have possibilities in energy conservation systems.
* Mischmetal
Mischmetal (from german: Mischmetall – "mixed metal") is an alloy of rare-earth elements. It is also called cerium mischmetal, or rare-earth mischmetal. A typical composition includes approximately 55% cerium, 25% lanthanum, and 15 ...
, a pyrophoric
A substance is pyrophoric (from grc-gre, πυροφόρος, , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolith ...
alloy used in lighter flints, contains 25% to 45% lanthanum.
* Lanthanum oxide and the boride A boride is a compound between boron and a less electronegative element, for example silicon boride (SiB3 and SiB6). The borides are a very large group of compounds that are generally high melting and are covalent more than ionic in nature. Some bo ...
are used in electronic vacuum tube
A vacuum tube, electron tube, valve (British usage), or tube (North America), is a device that controls electric current flow in a high vacuum between electrodes to which an electric potential difference has been applied.
The type known as ...
s as hot cathode
In vacuum tubes and gas-filled tubes, a hot cathode or thermionic cathode is a cathode electrode which is heated to make it emit electrons due to thermionic emission. This is in contrast to a cold cathode, which does not have a heating elemen ...
materials with strong emissivity of electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have n ...
s. Crystals of are used in high-brightness, extended-life, thermionic electron emission sources for electron microscope
An electron microscope is a microscope that uses a beam of accelerated electrons as a source of illumination. As the wavelength of an electron can be up to 100,000 times shorter than that of visible light photons, electron microscopes have a hi ...
s and Hall-effect thrusters.
* Lanthanum trifluoride () is an essential component of a heavy fluoride glass named ZBLAN. This glass has superior transmittance in the infrared range and is therefore used for fiber-optical communication systems.
* Cerium-doped lanthanum bromide and lanthanum chloride are the recent inorganic scintillator
A scintillator is a material that exhibits scintillation, the property of luminescence, when excited by ionizing radiation. Luminescent materials, when struck by an incoming particle, absorb its energy and scintillate (i.e. re-emit the absorbe ...
s, which have a combination of high light yield, best energy resolution, and fast response. Their high yield converts into superior energy resolution; moreover, the light output is very stable and quite high over a very wide range of temperatures, making it particularly attractive for high-temperature applications. These scintillators are already widely used commercially in detectors of neutrons
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the nuclei of atoms. Since protons and neutrons behave ...
or gamma rays
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically ...
.
* Carbon arc lamp
An arc lamp or arc light is a lamp that produces light by an electric arc (also called a voltaic arc).
The carbon arc light, which consists of an arc between carbon electrodes in air, invented by Humphry Davy in the first decade of the 1800s ...
s use a mixture of rare earth elements to improve the light quality. This application, especially by the motion picture
A film also called a movie, motion picture, moving picture, picture, photoplay or (slang) flick is a work of visual art that simulates experiences and otherwise communicates ideas, stories, perceptions, feelings, beauty, or atmosphere ...
industry for studio lighting and projection, consumed about 25% of the rare-earth compounds produced until the phase out of carbon arc lamps.
* Lanthanum(III) oxide () improves the alkali resistance of glass
Glass is a non- crystalline, often transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling (quenchin ...
and is used in making special optical glasses, such as infrared-absorbing glass, as well as camera
A camera is an optical instrument that can capture an image. Most cameras can capture 2D images, with some more advanced models being able to capture 3D images. At a basic level, most cameras consist of sealed boxes (the camera body), with ...
and telescope
A telescope is a device used to observe distant objects by their emission, absorption, or reflection of electromagnetic radiation. Originally meaning only an optical instrument using lenses, curved mirrors, or a combination of both to obse ...
lenses, because of the high refractive index
In optics, the refractive index (or refraction index) of an optical medium is a dimensionless number that gives the indication of the light bending ability of that medium.
The refractive index determines how much the path of light is bent, ...
and low dispersion of rare-earth glasses. Lanthanum oxide is also used as a grain-growth additive during the liquid-phase sintering
Clinker nodules produced by sintering
Sintering or frittage is the process of compacting and forming a solid mass of material by pressure or heat without melting it to the point of liquefaction.
Sintering happens as part of a manufacturing ...
of silicon nitride
Silicon nitride is a chemical compound of the elements silicon and nitrogen. is the most thermodynamically stable and commercially important of the silicon nitrides, and the term "silicon nitride" commonly refers to this specific composition. It ...
and zirconium diboride
Zirconium diboride (ZrB2) is a highly covalent refractory ceramic material with a hexagonal crystal structure. ZrB2 is an ultra high temperature ceramic (UHTC) with a melting point of 3246 °C. This along with its relatively low density of ...
.
* Small amounts of lanthanum added to steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistan ...
improves its malleability
Ductility is a mechanical property commonly described as a material's amenability to drawing (e.g. into wire). In materials science, ductility is defined by the degree to which a material can sustain plastic deformation under tensile stres ...
, resistance to impact, and ductility
Ductility is a mechanical property commonly described as a material's amenability to drawing (e.g. into wire). In materials science, ductility is defined by the degree to which a material can sustain plastic deformation under tensile str ...
, whereas addition of lanthanum to molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lead ...
decreases its hardness and sensitivity to temperature variations.
* Small amounts of lanthanum are present in many pool products to remove the phosphates that feed algae.
* Lanthanum oxide additive to tungsten is used in gas tungsten arc welding
Gas tungsten arc welding (GTAW), also known as tungsten inert gas (TIG) welding, is an arc welding process that uses a non-consumable tungsten electrode to produce the weld. The weld area and electrode are protected from oxidation or other atm ...
electrodes, as a substitute for radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi ...
thorium.
* Various compounds of lanthanum and other rare-earth elements (oxides, chlorides, etc.) are components of various catalysis, such as petroleum cracking catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
s.
* Lanthanum-barium radiometric dating
Radiometric dating, radioactive dating or radioisotope dating is a technique which is used to date materials such as rocks or carbon, in which trace radioactive impurities were selectively incorporated when they were formed. The method compares ...
is used to estimate age of rocks and ores, though the technique has limited popularity.
* Lanthanum carbonate was approved as a medication (Fosrenol, Shire Pharmaceuticals
Shire plc was a UK-founded Jersey-registered specialty biopharmaceutical company. Originating in the United Kingdom with an operational base in the United States, its brands and products included Vyvanse, Lialda, and Adderall XR. Shire was ...
) to absorb excess phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phosph ...
in cases of hyperphosphatemia
Hyperphosphatemia is an electrolyte disorder in which there is an elevated level of phosphate in the blood. Most people have no symptoms while others develop calcium deposits in the soft tissue. Often there is also low calcium levels which can ...
seen in end-stage kidney disease
Chronic kidney disease (CKD) is a type of kidney disease in which a gradual loss of kidney function occurs over a period of months to years. Initially generally no symptoms are seen, but later symptoms may include leg swelling, feeling tired, vo ...
.
* Lanthanum fluoride is used in phosphor lamp coatings. Mixed with europium fluoride, it is also applied in the crystal membrane of fluoride ion-selective electrodes.
* Like horseradish peroxidase
The enzyme horseradish peroxidase (HRP), found in the roots of horseradish, is used extensively in biochemistry applications. It is a metalloenzyme with many isoforms, of which the most studied type is C. It catalyzes the oxidation of various o ...
, lanthanum is used as an electron-dense tracer in molecular biology
Molecular biology is the branch of biology that seeks to understand the molecular basis of biological activity in and between cells, including biomolecular synthesis, modification, mechanisms, and interactions. The study of chemical and phys ...
.
* Lanthanum-modified bentonite (or phoslock Phoslock is the commercial name for a bentonite clay in which the sodium and/or calcium ions are exchanged for lanthanum. The lanthanum contained within Phoslock reacts with phosphate to form an inert mineral known as rhabdophane (LaPO4.\mathitH2O). ...
) is used to remove phosphates from water in lake treatments.
* Lanthanum telluride (La3Te4) is considered to be applied in the field of radioisotope power system (nuclear power plant) due to its significant conversion capabilities. The transmuted elements and isotopes in the segment will not react with the material itself, thus presenting no harm to the safety of the power plant. Though iodine, which can be generated during transmutation, is suspected to react with La3Te4 segment, the quantity of iodine is small enough to possess threat to the power system.
Biological role
Lanthanum has no known biological role in humans. The element is very poorly absorbed after oral administration and when injected its elimination is very slow. Lanthanum carbonate (Fosrenol) was approved as aphosphate binder Phosphate binders are medications used to reduce the absorption of dietary phosphate; they are taken along with meals and snacks. They are frequently used in people with chronic kidney failure (CKF), who are less able to excrete phosphate, resulting ...
to absorb excess phosphate in cases of end stage renal disease.
While lanthanum has pharmacological effects on several receptors and ion channels, its specificity for the GABA receptor is unique among trivalent cations. Lanthanum acts at the same modulatory site on the GABA receptor
The GABA receptors are a class of receptors that respond to the neurotransmitter gamma-aminobutyric acid (GABA), the chief inhibitory compound in the mature vertebrate central nervous system. There are two classes of GABA receptors: GABAA and ...
as zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodi ...
, a known negative allosteric
In biochemistry, allosteric regulation (or allosteric control) is the regulation of an enzyme by binding an effector molecule at a site other than the enzyme's active site.
The site to which the effector binds is termed the ''allosteric sit ...
modulator. The lanthanum cation La3+ is a positive allosteric modulator at native and recombinant GABA receptors, increasing open channel time and decreasing desensitization in a subunit configuration dependent manner.
Lanthanum is an essential cofactor for the methanol dehydrogenase of the methanotrophic bacterium ''Methylacidiphilum fumariolicum
''Methylacidiphilum fumariolicum '' is an autotrophic bacterium first described in 2007 growing on volcanic pools near Naples, Italy. It grows in mud at temperatures between 50 °C and 60 °C and an acidic pH of 2–5. It is able to ox ...
'' SolV, although the great chemical similarity of the lanthanides means that it may be substituted with cerium, praseodymium, or neodymium without ill effects, and with the smaller samarium, europium, or gadolinium giving no side effects other than slower growth.
Precautions
Lanthanum has a low to moderate level of toxicity and should be handled with care. The injection of lanthanum solutions produceshyperglycemia
Hyperglycemia is a condition in which an excessive amount of glucose circulates in the blood plasma. This is generally a blood sugar level higher than 11.1 mmol/L (200 mg/dL), but symptoms may not start to become noticeable until even ...
, low blood pressure, degeneration of the spleen
The spleen is an organ found in almost all vertebrates. Similar in structure to a large lymph node, it acts primarily as a blood filter. The word spleen comes .
and hepatic alterations. The application in carbon arc light led to the exposure of people to rare earth element oxides and fluorides, which sometimes led to pneumoconiosis
Pneumoconiosis is the general term for a class of interstitial lung disease where inhalation of dust ( for example, ash dust, lead particles, pollen grains etc) has caused interstitial fibrosis. The three most common types are asbestosis, silico ...
. As the La3+ ion is similar in size to the Ca2+ ion, it is sometimes used as an easily traced substitute for the latter in medical studies. Lanthanum, like the other lanthanides, is known to affect human metabolism, lowering cholesterol levels, blood pressure, appetite, and risk of blood coagulation. When injected into the brain, it acts as a painkiller, similarly to morphine
Morphine is a strong opiate that is found naturally in opium, a dark brown resin in poppies ('' Papaver somniferum''). It is mainly used as a pain medication, and is also commonly used recreationally, or to make other illicit opioids. T ...
and other opiates, though the mechanism behind this is still unknown.
Prices
The price for a (metric) ton 000 kgof ''Lanthanum oxide 99% (FOB China in USD/Mt)'' is given by the Institute of Rare Earths Elements and Strategic Metals as below $2,000 for most of the period from early 2001 to September 2010 (at $10,000 in the short term in 2008); it rose steeply to $140,000 in mid-2011 and fell back just as rapidly to $38,000 by early 2012. The average price for the last six months (April to September 2022) is given by the Institute as follows: ''Lanthanum Oxide - 99.9%min FOB China - 1308 EUR/mt'' and for ''Lanthanum Metal - 99%min FOB China - 3706 EUR/mt''.Information and notation: .access-date=27 October 2022.See also
, CASNo_Ref = , CASNo = 7439-91-0 , UNII_Ref = , UNII = 6I3K30563SReferences
Bibliography
*Further reading
* ''The Industrial Chemistry of the Lanthanons, Yttrium, Thorium and Uranium'', by R. J. Callow, Pergamon Press, 1967 * ''Extractive Metallurgy of Rare Earths'', by C. K. Gupta and N. Krishnamurthy, CRC Press, 2005 * ''Nouveau Traite de Chimie Minerale, Vol. VII. Scandium, Yttrium, Elements des Terres Rares, Actinium'', P. Pascal, Editor, Masson & Cie, 1959 * ''Chemistry of the Lanthanons'', by R. C. Vickery, Butterworths 1953 {{Good article Chemical elements Chemical elements with double hexagonal close-packed structure Lanthanides Reducing agents GABAA receptor positive allosteric modulators