
Glycolysis is the
metabolic pathway
In biochemistry, a metabolic pathway is a linked series of chemical reactions occurring within a cell. The reactants, products, and intermediates of an enzymatic reaction are known as metabolites, which are modified by a sequence of chemical reac ...
that converts
glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, u ...
() into
pyruvate (). The
free energy released in this process is used to form the high-energy molecules
adenosine triphosphate
Adenosine triphosphate (ATP) is an organic compound that provides energy to drive many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms o ...
(ATP) and
reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by
enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
s.

Glycolysis is a metabolic pathway that does not require oxygen (In anaerobic conditions pyruvate is converted to lactic acid). The wide occurrence of glycolysis in other species indicates that it is an ancient metabolic pathway. Indeed, the reactions that make up glycolysis and its parallel pathway, the
pentose phosphate pathway
The pentose phosphate pathway (also called the phosphogluconate pathway and the hexose monophosphate shunt and the HMP Shunt) is a metabolic pathway parallel to glycolysis. It generates NADPH and pentoses (5-carbon sugars) as well as ribose 5-pho ...
, occur in the
oxygen-free conditions of the
Archean
The Archean Eon ( , also spelled Archaean or Archæan) is the second of four geologic eons of Earth's history, representing the time from . The Archean was preceded by the Hadean Eon and followed by the Proterozoic.
The Earth during the Arc ...
oceans, also in the absence of enzymes, catalyzed by metal.
In most organisms, glycolysis occurs in the liquid part of cells, the
cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells ( intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondri ...
. The most common type of glycolysis is the ''Embden–Meyerhof–Parnas (EMP) pathway'', which was discovered by
Gustav Embden
Gustav Georg Embden (10 November 1874 – 25 July 1933) was a German physiological chemist.
Background
Gustav Embden was a son of the Hamburg lawyer and politician George Heinrich Embden. His grandmother Charlotte Heine was a well-known salonn ...
,
Otto Meyerhof
Otto Fritz Meyerhof (; April 12, 1884 – October 6, 1951) was a German physician and biochemist who won the 1922 Nobel Prize in Physiology and Medicine.
Biography
Otto Fritz Meyerhof was born in Hannover, at Theaterplatz 16A (now:Rathenaustrasse ...
, and
Jakub Karol Parnas
Jakub Karol Parnas, also known as Yakov Oskarovich Parnas (russian: Яков Оскарович Парнас) (January 16, 1884 – January 29, 1949) was a prominent Polish–Soviet biochemist who contributed to the discovery of the Embden� ...
. Glycolysis also refers to other pathways, such as the ''
Entner–Doudoroff pathway
The Entner–Doudoroff pathway (ED Pathway) is a metabolic pathway that is most notable in Gram-negative bacteria, certain Gram-positive bacteria and archaea. Glucose is the substrate in the ED pathway and through a series of enzyme assisted chem ...
'' and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.
The glycolysis pathway can be separated into two phases:
# Investment phase – wherein ATP is consumed
# Yield phase – wherein more ATP is produced than originally consumed
Overview
The overall reaction of glycolysis is:

The use of symbols in this equation makes it appear unbalanced with respect to oxygen atoms, hydrogen atoms, and charges. Atom balance is maintained by the two phosphate (P
i) groups:
* Each exists in the form of a
hydrogen phosphate anion (), dissociating to contribute overall
* Each liberates an oxygen atom when it binds to an
adenosine diphosphate (ADP) molecule, contributing 2O overall
Charges are balanced by the difference between ADP and ATP. In the cellular environment, all three hydroxyl groups of ADP dissociate into −O
− and H
+, giving ADP
3−, and this ion tends to exist in an ionic bond with Mg
2+, giving ADPMg
−. ATP behaves identically except that it has four hydroxyl groups, giving ATPMg
2−. When these differences along with the true charges on the two phosphate groups are considered together, the net charges of −4 on each side are balanced.
For simple
fermentations, the metabolism of one molecule of glucose to two molecules of pyruvate has a net yield of two molecules of ATP. Most cells will then carry out further reactions to "repay" the used NAD
+ and produce a final product of
ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
or
lactic acid
Lactic acid is an organic acid. It has a molecular formula . It is white in the solid state and it is miscible with water. When in the dissolved state, it forms a colorless solution. Production includes both artificial synthesis as well as nat ...
. Many bacteria use inorganic compounds as hydrogen acceptors to regenerate the NAD
+.
Cells performing
aerobic respiration synthesize much more ATP, but not as part of glycolysis. These further aerobic reactions use
pyruvate, and NADH + H
+ from glycolysis. Eukaryotic aerobic respiration produces approximately 34 additional molecules of ATP for each glucose molecule, however most of these are produced by a mechanism vastly different from the
substrate-level phosphorylation in glycolysis.
The lower-energy production, per glucose, of anaerobic respiration relative to aerobic respiration, results in greater flux through the pathway under hypoxic (low-oxygen) conditions, unless alternative sources of anaerobically oxidizable substrates, such as fatty acids, are found.
History
The pathway of glycolysis as it is known today took almost 100 years to fully elucidate.
The combined results of many smaller experiments were required in order to understand the pathway as a whole.
The first steps in understanding glycolysis began in the nineteenth century with the wine industry. For economic reasons, the French wine industry sought to investigate why wine sometimes turned distasteful, instead of fermenting into alcohol. French scientist
Louis Pasteur researched this issue during the 1850s, and the results of his experiments began the long road to elucidating the pathway of glycolysis. His experiments showed that fermentation occurs by the action of living
microorganism
A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, ὀργανισμός, ''organismós'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in olde ...
s, yeasts, and that yeast's glucose consumption decreased under aerobic conditions of fermentation, in comparison to anaerobic conditions (the
Pasteur effect).

Insight into the component steps of glycolysis were provided by the non-cellular fermentation experiments of
Eduard Buchner
Eduard Buchner (; 20 May 1860 – 13 August 1917) was a German chemist and zymologist, awarded the 1907 Nobel Prize in Chemistry for his work on fermentation.
Biography
Early years
Buchner was born in Munich to a physician and Doctor Extraor ...
during the 1890s. Buchner demonstrated that the conversion of glucose to ethanol was possible using a non-living extract of yeast, due to the action of
enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products ...
s in the extract.
This experiment not only revolutionized biochemistry, but also allowed later scientists to analyze this pathway in a more controlled laboratory setting. In a series of experiments (1905-1911), scientists
Arthur Harden
Sir Arthur Harden, FRS (12 October 1865 – 17 June 1940) was a British biochemist. He shared the Nobel Prize in Chemistry in 1929 with Hans Karl August Simon von Euler-Chelpin for their investigations into the fermentation of sugar and ferment ...
and
William Young discovered more pieces of glycolysis.
They discovered the regulatory effects of ATP on glucose consumption during alcohol fermentation. They also shed light on the role of one compound as a glycolysis intermediate: fructose 1,6-bisphosphate.
The elucidation of fructose 1,6-bisphosphate was accomplished by measuring levels when yeast juice was incubated with glucose. production increased rapidly then slowed down. Harden and Young noted that this process would restart if an inorganic phosphate (Pi) was added to the mixture. Harden and Young deduced that this process produced organic phosphate esters, and further experiments allowed them to extract fructose diphosphate (F-1,6-DP).
Arthur Harden
Sir Arthur Harden, FRS (12 October 1865 – 17 June 1940) was a British biochemist. He shared the Nobel Prize in Chemistry in 1929 with Hans Karl August Simon von Euler-Chelpin for their investigations into the fermentation of sugar and ferment ...
and
William Young along with Nick Sheppard determined, in a second experiment, that a heat-sensitive high-molecular-weight subcellular fraction (the enzymes) and a heat-insensitive low-molecular-weight cytoplasm fraction (ADP, ATP and NAD
+ and other
cofactors) are required together for fermentation to proceed. This experiment begun by observing that dialyzed (purified) yeast juice could not ferment or even create a sugar phosphate. This mixture was rescued with the addition of undialyzed yeast extract that had been boiled. Boiling the yeast extract renders all proteins inactive (as it denatures them). The ability of boiled extract plus dialyzed juice to complete fermentation suggests that the cofactors were non-protein in character.

In the 1920s
Otto Meyerhof
Otto Fritz Meyerhof (; April 12, 1884 – October 6, 1951) was a German physician and biochemist who won the 1922 Nobel Prize in Physiology and Medicine.
Biography
Otto Fritz Meyerhof was born in Hannover, at Theaterplatz 16A (now:Rathenaustrasse ...
was able to link together some of the many individual pieces of glycolysis discovered by Buchner, Harden, and Young. Meyerhof and his team were able to extract different glycolytic enzymes from
muscle tissue
Muscle tissue (or muscular tissue) is soft tissue that makes up the different types of muscles in most animals, and give the ability of muscles to contract. Muscle tissue is formed during embryonic development, in a process known as myogenesis. ...
, and combine them to artificially create the pathway from glycogen to lactic acid.
In one paper, Meyerhof and scientist Renate Junowicz-Kockolaty investigated the reaction that splits fructose 1,6-diphosphate into the two triose phosphates. Previous work proposed that the split occurred via 1,3-diphosphoglyceraldehyde plus an oxidizing enzyme and cozymase. Meyerhoff and Junowicz found that the equilibrium constant for the isomerase and aldoses reaction were not affected by inorganic phosphates or any other cozymase or oxidizing enzymes. They further removed diphosphoglyceraldehyde as a possible intermediate in glycolysis.
With all of these pieces available by the 1930s,
Gustav Embden
Gustav Georg Embden (10 November 1874 – 25 July 1933) was a German physiological chemist.
Background
Gustav Embden was a son of the Hamburg lawyer and politician George Heinrich Embden. His grandmother Charlotte Heine was a well-known salonn ...
proposed a detailed, step-by-step outline of that pathway we now know as glycolysis. The biggest difficulties in determining the intricacies of the pathway were due to the very short lifetime and low steady-state concentrations of the intermediates of the fast glycolytic reactions. By the 1940s, Meyerhof, Embden and many other biochemists had finally completed the puzzle of glycolysis.
The understanding of the isolated pathway has been expanded in the subsequent decades, to include further details of its regulation and integration with other metabolic pathways.
Sequence of reactions
Summary of reactions
Preparatory phase
The first five steps of Glycolysis are regarded as the preparatory (or investment) phase, since they consume energy to convert the glucose into two three-carbon sugar phosphates
(
G3P).
The first step is phosphorylation of glucose by a family of enzymes called
hexokinase
A hexokinase is an enzyme that phosphorylates hexoses (six-carbon sugars), forming hexose phosphate. In most organisms, glucose is the most important substrate for hexokinases, and glucose-6-phosphate is the most important product. Hexok ...
s to form glucose 6-phosphate (G6P). This reaction consumes ATP, but it acts to keep the glucose concentration low, promoting continuous transport of glucose into the cell through the plasma membrane transporters. In addition, it blocks the glucose from leaking out – the cell lacks transporters for G6P, and free diffusion out of the cell is prevented due to the charged nature of G6P. Glucose may alternatively be formed from the
phosphorolysis Phosphorolysis is the cleavage of a compound in which inorganic phosphate is the attacking group. It is analogous to hydrolysis.Stryer, L. (1988) Biochemistry, 3rd ed., Freeman (p. 451)
An example of this is glycogen breakdown by glycogen phospho ...
or
hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolys ...
of intracellular starch or glycogen.
In
animal
Animals are multicellular, eukaryotic organisms in the Kingdom (biology), biological kingdom Animalia. With few exceptions, animals Heterotroph, consume organic material, Cellular respiration#Aerobic respiration, breathe oxygen, are Motilit ...
s, an
isozyme In biochemistry, isozymes (also known as isoenzymes or more generally as multiple forms of enzymes) are enzymes that differ in amino acid sequence but catalyze the same chemical reaction. Isozymes usually have different kinetic parameters (e.g. dif ...
of hexokinase called
glucokinase
Glucokinase () is an enzyme that facilitates phosphorylation of glucose to glucose-6-phosphate. Glucokinase occurs in cells in the liver and pancreas of humans and most other vertebrates. In each of these organs it plays an important role i ...
is also used in the liver, which has a much lower affinity for glucose (K
m in the vicinity of normal glycemia), and differs in regulatory properties. The different substrate affinity and alternate regulation of this enzyme are a reflection of the role of the liver in maintaining blood sugar levels.
''Cofactors:'' Mg
2+
G6P is then rearranged into
fructose 6-phosphate
Fructose 6-phosphate (sometimes called the Neuberg ester) is a derivative of fructose, which has been phosphorylated at the 6-hydroxy group. It is one of several possible fructosephosphates. The β-D-form of this compound is very common in cells. ...
(F6P) by
glucose phosphate isomerase.
Fructose can also enter the glycolytic pathway by phosphorylation at this point.
The change in structure is an isomerization, in which the G6P has been converted to F6P. The reaction requires an enzyme, phosphoglucose isomerase, to proceed. This reaction is freely reversible under normal cell conditions. However, it is often driven forward because of a low concentration of F6P, which is constantly consumed during the next step of glycolysis. Under conditions of high F6P concentration, this reaction readily runs in reverse. This phenomenon can be explained through
Le Chatelier's Principle
Le Chatelier's principle (pronounced or ), also called Chatelier's principle (or the Equilibrium Law), is a principle of chemistry used to predict the effect of a change in conditions on chemical equilibria. The principle is named after French c ...
. Isomerization to a keto sugar is necessary for carbanion stabilization in the fourth reaction step (below).
The energy expenditure of another ATP in this step is justified in 2 ways: The glycolytic process (up to this step) becomes irreversible, and the energy supplied destabilizes the molecule. Because the reaction catalyzed by
phosphofructokinase 1
Phosphofructokinase-1 (PFK-1) is one of the most important regulatory enzymes () of glycolysis. It is an allosteric enzyme made of 4 subunits and controlled by many activators and inhibitors. PFK-1 catalyzes the important "committed" step of gl ...
(PFK-1) is coupled to the hydrolysis of ATP (an energetically favorable step) it is, in essence, irreversible, and a different pathway must be used to do the reverse conversion during
gluconeogenesis. This makes the reaction a key regulatory point (see below).
Furthermore, the second phosphorylation event is necessary to allow the formation of two charged groups (rather than only one) in the subsequent step of glycolysis, ensuring the prevention of free diffusion of substrates out of the cell.
The same reaction can also be catalyzed by
pyrophosphate-dependent phosphofructokinase (PFP or PPi-PFK), which is found in most plants, some bacteria, archea, and protists, but not in animals. This enzyme uses pyrophosphate (PPi) as a phosphate donor instead of ATP. It is a reversible reaction, increasing the flexibility of glycolytic metabolism. A rarer ADP-dependent PFK enzyme variant has been identified in archaean species.
''Cofactors:'' Mg
2+
Destabilizing the molecule in the previous reaction allows the hexose ring to be split by
aldolase
Fructose-bisphosphate aldolase (), often just aldolase, is an enzyme catalyzing a reversible reaction that splits the aldol, fructose 1,6-bisphosphate, into the triose phosphates dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phospha ...
into two triose sugars:
dihydroxyacetone phosphate
Dihydroxyacetone phosphate (DHAP, also glycerone phosphate in older texts) is the anion with the formula HOCH2C(O)CH2OPO32-. This anion is involved in many metabolic pathways, including the Calvin cycle in plants and glycolysis.Nelson, D. L.; Co ...
(a ketose), and
glyceraldehyde 3-phosphate
Glyceraldehyde 3-phosphate, also known as triose phosphate or 3-phosphoglyceraldehyde and abbreviated as G3P, GA3P, GADP, GAP, TP, GALP or PGAL, is a metabolite that occurs as an intermediate in several central pathways of all organisms.Nelson, D ...
(an aldose). There are two classes of aldolases: class I aldolases, present in animals and plants, and class II aldolases, present in fungi and bacteria; the two classes use different mechanisms in cleaving the ketose ring.
Electrons delocalized in the carbon-carbon bond cleavage associate with the alcohol group. The resulting carbanion is stabilized by the structure of the carbanion itself via resonance charge distribution and by the presence of a charged ion prosthetic group.
Triosephosphate isomerase
Triose-phosphate isomerase (TPI or TIM) is an enzyme () that catalyzes the reversible interconversion of the triose phosphate isomers dihydroxyacetone phosphate and D-glyceraldehyde 3-phosphate.
TPI plays an important role in glycolysis and ...
rapidly interconverts dihydroxyacetone phosphate with
glyceraldehyde 3-phosphate
Glyceraldehyde 3-phosphate, also known as triose phosphate or 3-phosphoglyceraldehyde and abbreviated as G3P, GA3P, GADP, GAP, TP, GALP or PGAL, is a metabolite that occurs as an intermediate in several central pathways of all organisms.Nelson, D ...
(GADP) that proceeds further into glycolysis. This is advantageous, as it directs dihydroxyacetone phosphate down the same pathway as glyceraldehyde 3-phosphate, simplifying regulation.
Pay-off phase
The second half of glycolysis is known as the pay-off phase, characterised by a net gain of the energy-rich molecules ATP and NADH.
Since glucose leads to two triose sugars in the preparatory phase, each reaction in the pay-off phase occurs twice per glucose molecule. This yields 2 NADH molecules and 4 ATP molecules, leading to a net gain of 2 NADH molecules and 2 ATP molecules from the glycolytic pathway per glucose.
The aldehyde groups of the triose sugars are
oxidised
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a d ...
, and
inorganic phosphate
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid .
The phosphate or orthophosphate ion is derived from phosph ...
is added to them, forming
1,3-bisphosphoglycerate.
The hydrogen is used to reduce two molecules of
NAD+, a hydrogen carrier, to give NADH + H
+ for each triose.
Hydrogen atom balance and charge balance are both maintained because the phosphate (P
i) group actually exists in the form of a
hydrogen phosphate anion (),
which dissociates to contribute the extra H
+ ion and gives a net charge of -3 on both sides.
Here,
arsenate
The arsenate ion is .
An arsenate (compound) is any compound that contains this ion. Arsenates are salts or esters of arsenic acid.
The arsenic atom in arsenate has a valency of 5 and is also known as pentavalent arsenic or As(V).
Arsenate res ...
(), an anion akin to inorganic phosphate may replace phosphate as a substrate to form 1-arseno-3-phosphoglycerate. This, however, is unstable and readily hydrolyzes to form
3-phosphoglycerate
3-Phosphoglyceric acid (3PG, 3-PGA, or PGA) is the conjugate acid of 3-phosphoglycerate or glycerate 3-phosphate (GP or G3P). This glycerate is a biochemically significant metabolic intermediate in both glycolysis and the Calvin-Benson cycle. The ...
, the intermediate in the next step of the pathway. As a consequence of bypassing this step, the molecule of ATP generated from
1-3 bisphosphoglycerate in the next reaction will not be made, even though the reaction proceeds. As a result, arsenate is an uncoupler of glycolysis.
This step is the enzymatic transfer of a phosphate group from
1,3-bisphosphoglycerate to ADP by
phosphoglycerate kinase
Phosphoglycerate kinase () (PGK 1) is an enzyme that catalyzes the reversible transfer of a phosphate group from 1,3-bisphosphoglycerate (1,3-BPG) to ADP producing 3-phosphoglycerate (3-PG) and ATP :
:1,3-bisphosphoglycerate + ADP glycerat ...
, forming ATP and
3-phosphoglycerate
3-Phosphoglyceric acid (3PG, 3-PGA, or PGA) is the conjugate acid of 3-phosphoglycerate or glycerate 3-phosphate (GP or G3P). This glycerate is a biochemically significant metabolic intermediate in both glycolysis and the Calvin-Benson cycle. The ...
. At this step, glycolysis has reached the break-even point: 2 molecules of ATP were consumed, and 2 new molecules have now been synthesized. This step, one of the two
substrate-level phosphorylation steps, requires ADP; thus, when the cell has plenty of ATP (and little ADP), this reaction does not occur. Because ATP decays relatively quickly when it is not metabolized, this is an important regulatory point in the glycolytic pathway.
ADP actually exists as ADPMg
−, and ATP as ATPMg
2−, balancing the charges at −5 both sides.
''Cofactors:'' Mg
2+
Phosphoglycerate mutase
:''This enzyme is not to be confused with Bisphosphoglycerate mutase which catalyzes the conversion of 1,3-bisphosphoglycerate to 2,3-bisphosphoglycerate.''
Phosphoglycerate mutase (PGM) is any enzyme that catalyzes step 8 of glycolysis - ...
isomerises
3-phosphoglycerate
3-Phosphoglyceric acid (3PG, 3-PGA, or PGA) is the conjugate acid of 3-phosphoglycerate or glycerate 3-phosphate (GP or G3P). This glycerate is a biochemically significant metabolic intermediate in both glycolysis and the Calvin-Benson cycle. The ...
into
2-phosphoglycerate.
Enolase
Phosphopyruvate hydratase, usually known as enolase, is a metalloenzyme () that catalyses the conversion of 2-phosphoglycerate (2-PG) to phosphoenolpyruvate (PEP), the ninth and penultimate step of glycolysis. The chemical reaction is:
:2-p ...
next converts
2-phosphoglycerate to
phosphoenolpyruvate
Phosphoenolpyruvate (2-phosphoenolpyruvate, PEP) is the ester derived from the enol of pyruvate and phosphate. It exists as an anion. PEP is an important intermediate in biochemistry. It has the highest-energy phosphate bond found (−61.9 kJ/m ...
. This reaction is an elimination reaction involving an
E1cB mechanism.
''Cofactors:'' 2 Mg
2+, one "conformational" ion to coordinate with the carboxylate group of the substrate, and one "catalytic" ion that participates in the dehydration.
A final
substrate-level phosphorylation now forms a molecule of
pyruvate and a molecule of ATP by means of the enzyme
pyruvate kinase
Pyruvate kinase is the enzyme involved in the last step of glycolysis. It catalyzes the transfer of a phosphate group from phosphoenolpyruvate (PEP) to adenosine diphosphate (ADP), yielding one molecule of pyruvate and one molecule of ATP. P ...
. This serves as an additional regulatory step, similar to the phosphoglycerate kinase step.
''Cofactors:'' Mg
2+
Biochemical logic
The existence of more than one point of regulation indicates that intermediates between those points enter and leave the glycolysis pathway by other processes. For example, in the first regulated step,
hexokinase
A hexokinase is an enzyme that phosphorylates hexoses (six-carbon sugars), forming hexose phosphate. In most organisms, glucose is the most important substrate for hexokinases, and glucose-6-phosphate is the most important product. Hexok ...
converts glucose into glucose-6-phosphate. Instead of continuing through the glycolysis pathway, this intermediate can be converted into glucose storage molecules, such as
glycogen or
starch. The reverse reaction, breaking down, e.g., glycogen, produces mainly glucose-6-phosphate; very little free glucose is formed in the reaction. The glucose-6-phosphate so produced can enter glycolysis ''after'' the first control point.
In the second regulated step (the third step of glycolysis),
phosphofructokinase
Phosphofructokinase (PFK) is a kinase enzyme that phosphorylates fructose 6-phosphate in glycolysis.
Function
The enzyme-catalysed transfer of a phosphoryl group from ATP is an important reaction in a wide variety of biological processes. ...
converts fructose-6-phosphate into fructose-1,6-bisphosphate, which then is converted into glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. The dihydroxyacetone phosphate can be removed from glycolysis by conversion into glycerol-3-phosphate, which can be used to form triglycerides. Conversely,
triglyceride
A triglyceride (TG, triacylglycerol, TAG, or triacylglyceride) is an ester derived from glycerol and three fatty acids (from ''wikt:tri-#Prefix, tri-'' and ''glyceride'').
Triglycerides are the main constituents of body fat in humans and other ...
s can be broken down into fatty acids and glycerol; the latter, in turn, can be
converted into dihydroxyacetone phosphate, which can enter glycolysis ''after'' the second control point.
Free energy changes
The change in free energy, Δ''G'', for each step in the glycolysis pathway can be calculated using Δ''G'' = Δ''G''°' + ''RT''ln ''Q'', where ''Q'' is the
reaction quotient In chemical thermodynamics, the reaction quotient (''Q''r or just ''Q'') is a dimensionless quantity that provides a measurement of the relative amounts of products and reactants present in a reaction mixture for a reaction with well-defined overall ...
. This requires knowing the concentrations of the
metabolites
In biochemistry, a metabolite is an intermediate or end product of metabolism.
The term is usually used for small molecules. Metabolites have various functions, including fuel, structure, signaling, stimulatory and inhibitory effects on enzymes, ...
. All of these values are available for
erythrocytes
Red blood cells (RBCs), also referred to as red cells, red blood corpuscles (in humans or other animals not having nucleus in red blood cells), haematids, erythroid cells or erythrocytes (from Greek ''erythros'' for "red" and ''kytos'' for "holl ...
, with the exception of the concentrations of NAD
+ and NADH. The ratio of
NAD+ to NADH in the cytoplasm is approximately 1000, which makes the oxidation of glyceraldehyde-3-phosphate (step 6) more favourable.
Using the measured concentrations of each step, and the standard free energy changes, the actual free energy change can be calculated. (Neglecting this is very common - the delta G of ATP hydrolysis in cells is not the standard free energy change of ATP hydrolysis quoted in textbooks).
From measuring the physiological concentrations of metabolites in an erythrocyte it seems that about seven of the steps in glycolysis are in equilibrium for that cell type. Three of the steps — the ones with large negative free energy changes — are not in equilibrium and are referred to as ''irreversible''; such steps are often subject to regulation.
Step 5 in the figure is shown behind the other steps, because that step is a side-reaction that can decrease or increase the concentration of the intermediate glyceraldehyde-3-phosphate. That compound is converted to dihydroxyacetone phosphate by the enzyme triose phosphate isomerase, which is a
catalytically perfect enzyme; its rate is so fast that the reaction can be assumed to be in equilibrium. The fact that Δ''G'' is not zero indicates that the actual concentrations in the erythrocyte are not accurately known.
Regulation
The enzymes that catalyse glycolysis are regulated via a range of biological mechanisms in order to control overall
flux though the pathway. This is vital for both
homeostatsis in a static environment, and
metabolic adaptation to a changing environment or need. The details of regulation for some enzymes are highly conserved between species, whereas others vary widely.
# Gene Expression: Firstly, the cellular concentrations of glycolytic enzymes are modulated via
regulation of gene expression via
transcription factors
In molecular biology, a transcription factor (TF) (or sequence-specific DNA-binding factor) is a protein that controls the rate of transcription of genetic information from DNA to messenger RNA, by binding to a specific DNA sequence. The fun ...
, with several glycolysis enzymes themselves acting as
regulatory protein kinases in the nucleus.
#
Allosteric inhibition
In biochemistry, allosteric regulation (or allosteric control) is the regulation of an enzyme by binding an effector molecule at a site other than the enzyme's active site.
The site to which the effector binds is termed the ''allosteric site ...
and activation by metabolites: In particular
end-product inhibition
An enzyme inhibitor is a molecule that binds to an enzyme and blocks its activity. Enzymes are proteins that speed up chemical reactions necessary for life, in which substrate molecules are converted into products. An enzyme facilitates a sp ...
of regulated enzymes by metabolites such as ATP serves as negative feedback regulation of the pathway.
# Allosteric inhibition and activation by
Protein-protein interactions (PPI). Indeed, some proteins interact with and regulate multiple glycolytic enzymes.
#
Post-translational modification (PTM). In particular, phosphorylation and dephosphorylation is a key mechanism of regulation of pyruvate kinase in the liver.
#
Localization
Localization or localisation may refer to:
Biology
* Localization of function, locating psychological functions in the brain or nervous system; see Linguistic intelligence
* Localization of sensation, ability to tell what part of the body is a ...
Regulation by insulin in animals
In animals, regulation of blood glucose levels by the pancreas in conjunction with the liver is a vital part of
homeostasis
In biology, homeostasis (British also homoeostasis) (/hɒmɪə(ʊ)ˈsteɪsɪs/) is the state of steady internal, physical, and chemical conditions maintained by living systems. This is the condition of optimal functioning for the organism and ...
. The
beta cells
Beta cells (β-cells) are a type of cell found in pancreatic islets that synthesize and secrete insulin and amylin. Beta cells make up 50–70% of the cells in human islets. In patients with Type 1 diabetes, beta-cell mass and function are dim ...
in the
pancreatic islets
The pancreatic islets or islets of Langerhans are the regions of the pancreas that contain its endocrine (hormone-producing) cells, discovered in 1869 by German pathological anatomist Paul Langerhans. The pancreatic islets constitute 1–2% o ...
are sensitive to the blood glucose concentration.
A rise in the blood glucose concentration causes them to release
insulin into the blood, which has an effect particularly on the liver, but also on
fat
In nutrition, biology, and chemistry, fat usually means any ester of fatty acids, or a mixture of such compounds, most commonly those that occur in living beings or in food.
The term often refers specifically to triglycerides (triple est ...
and
muscle cells, causing these tissues to remove glucose from the blood. When the blood sugar falls the pancreatic beta cells cease insulin production, but, instead, stimulate the neighboring pancreatic
alpha cells
Alpha cells (α cells) are endocrine cells that are found in the Islets of Langerhans in the pancreas. Alpha cells secrete the peptide hormone glucagon in order to increase glucose levels in the blood stream.
Discovery
Islets of Langerhans were ...
to release
glucagon into the blood.
This, in turn, causes the liver to release glucose into the blood by breaking down stored
glycogen, and by means of gluconeogenesis. If the fall in the blood glucose level is particularly rapid or severe, other glucose sensors cause the release of
epinephrine from the
adrenal glands
The adrenal glands (also known as suprarenal glands) are endocrine glands that produce a variety of hormones including adrenaline and the steroids aldosterone and cortisol. They are found above the kidneys. Each gland has an outer cortex whic ...
into the blood. This has the same action as glucagon on glucose metabolism, but its effect is more pronounced.
In the liver glucagon and epinephrine cause the
phosphorylation of the key, regulated enzymes of glycolysis,
fatty acid synthesis
In biochemistry, fatty acid synthesis is the creation of fatty acids from acetyl-CoA and NADPH through the action of enzymes called fatty acid synthases. This process takes place in the cytoplasm of the cell. Most of the acetyl-CoA which is co ...
,
cholesterol synthesis
The mevalonate pathway, also known as the isoprenoid pathway or HMG-CoA reductase pathway is an essential metabolic pathway present in eukaryotes, archaea, and some bacteria. The pathway produces two five-carbon building blocks called isopenten ...
, gluconeogenesis, and glycogenolysis. Insulin has the opposite effect on these enzymes.
The phosphorylation and dephosphorylation of these enzymes (ultimately in response to the glucose level in the blood) is the dominant manner by which these pathways are controlled in the liver, fat, and muscle cells. Thus the phosphorylation of
phosphofructokinase
Phosphofructokinase (PFK) is a kinase enzyme that phosphorylates fructose 6-phosphate in glycolysis.
Function
The enzyme-catalysed transfer of a phosphoryl group from ATP is an important reaction in a wide variety of biological processes. ...
inhibits glycolysis, whereas its dephosphorylation through the action of insulin stimulates glycolysis.
Regulated Enzymes in Glycolysis
The three
regulatory enzymes are
hexokinase
A hexokinase is an enzyme that phosphorylates hexoses (six-carbon sugars), forming hexose phosphate. In most organisms, glucose is the most important substrate for hexokinases, and glucose-6-phosphate is the most important product. Hexok ...
(or
glucokinase
Glucokinase () is an enzyme that facilitates phosphorylation of glucose to glucose-6-phosphate. Glucokinase occurs in cells in the liver and pancreas of humans and most other vertebrates. In each of these organs it plays an important role i ...
in the liver),
phosphofructokinase
Phosphofructokinase (PFK) is a kinase enzyme that phosphorylates fructose 6-phosphate in glycolysis.
Function
The enzyme-catalysed transfer of a phosphoryl group from ATP is an important reaction in a wide variety of biological processes. ...
, and
pyruvate kinase
Pyruvate kinase is the enzyme involved in the last step of glycolysis. It catalyzes the transfer of a phosphate group from phosphoenolpyruvate (PEP) to adenosine diphosphate (ADP), yielding one molecule of pyruvate and one molecule of ATP. P ...
. The
flux through the glycolytic pathway is adjusted in response to conditions both inside and outside the cell. The internal factors that regulate glycolysis do so primarily to provide
ATP in adequate quantities for the cell's needs. The external factors act primarily on the
liver
The liver is a major organ only found in vertebrates which performs many essential biological functions such as detoxification of the organism, and the synthesis of proteins and biochemicals necessary for digestion and growth. In humans, it ...
,
fat tissue
Adipose tissue, body fat, or simply fat is a loose connective tissue composed mostly of adipocytes. In addition to adipocytes, adipose tissue contains the stromal vascular fraction (SVF) of cells including preadipocytes, fibroblasts, vascular en ...
, and
muscles, which can remove large quantities of glucose from the blood after meals (thus preventing
hyperglycemia by storing the excess glucose as fat or glycogen, depending on the tissue type). The liver is also capable of releasing glucose into the blood between meals, during fasting, and exercise thus preventing
hypoglycemia
Hypoglycemia, also called low blood sugar, is a fall in blood sugar to levels below normal, typically below 70 mg/dL (3.9 mmol/L). Whipple's triad is used to properly identify hypoglycemic episodes. It is defined as blood glucose bel ...
by means of
glycogenolysis and
gluconeogenesis. These latter reactions coincide with the halting of glycolysis in the liver.
In addition hexokinase and
glucokinase
Glucokinase () is an enzyme that facilitates phosphorylation of glucose to glucose-6-phosphate. Glucokinase occurs in cells in the liver and pancreas of humans and most other vertebrates. In each of these organs it plays an important role i ...
act independently of the hormonal effects as controls at the entry points of glucose into the cells of different tissues. Hexokinase responds to the
glucose-6-phosphate
Glucose 6-phosphate (G6P, sometimes called the Robison ester) is a glucose sugar phosphorylated at the hydroxy group on carbon 6. This dianion is very common in cells as the majority of glucose entering a cell will become phosphorylated in this wa ...
(G6P) level in the cell, or, in the case of glucokinase, to the blood sugar level in the blood to impart entirely intracellular controls of the glycolytic pathway in different tissues (see
below).
When glucose has been converted into G6P by hexokinase or glucokinase, it can either be converted to
glucose-1-phosphate
Glucose 1-phosphate (also called cori ester) is a glucose molecule with a phosphate group on the 1'-carbon. It can exist in either the α- or β- anomeric form.
Reactions of α-glucose 1-phosphate Catabolic
In glycogenolysis, it is the direct p ...
(G1P) for conversion to
glycogen, or it is alternatively converted by glycolysis to
pyruvate, which enters the
mitochondrion where it is converted into
acetyl-CoA and then into
citrate
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the ...
. Excess
citrate
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the ...
is exported from the mitochondrion back into the cytosol, where
ATP citrate lyase
ATP citrate synthase (also ATP citrate lyase (ACLY)) is an enzyme that in animals represents an important step in fatty acid biosynthesis. By converting citrate to acetyl-CoA, the enzyme links carbohydrate metabolism, which yields citrate as an ...
regenerates
acetyl-CoA and
oxaloacetate
Oxaloacetic acid (also known as oxalacetic acid or OAA) is a crystalline organic compound with the chemical formula HO2CC(O)CH2CO2H. Oxaloacetic acid, in the form of its conjugate base oxaloacetate, is a metabolic intermediate in many processes ...
(OAA). The acetyl-CoA is then used for
fatty acid synthesis
In biochemistry, fatty acid synthesis is the creation of fatty acids from acetyl-CoA and NADPH through the action of enzymes called fatty acid synthases. This process takes place in the cytoplasm of the cell. Most of the acetyl-CoA which is co ...
and
cholesterol synthesis
The mevalonate pathway, also known as the isoprenoid pathway or HMG-CoA reductase pathway is an essential metabolic pathway present in eukaryotes, archaea, and some bacteria. The pathway produces two five-carbon building blocks called isopenten ...
, two important ways of utilizing excess glucose when its concentration is high in blood. The regulated enzymes catalyzing these reactions perform these functions when they have been dephosphorylated through the action of insulin on the liver cells. Between meals, during
fasting
Fasting is the abstention from eating and sometimes drinking. From a purely physiological context, "fasting" may refer to the metabolic status of a person who has not eaten overnight (see " Breakfast"), or to the metabolic state achieved after ...
,
exercise or hypoglycemia, glucagon and epinephrine are released into the blood. This causes liver glycogen to be converted back to G6P, and then converted to glucose by the liver-specific enzyme
glucose 6-phosphatase
The enzyme glucose 6-phosphatase (EC 3.1.3.9, G6Pase; systematic name D-glucose-6-phosphate phosphohydrolase) catalyzes the hydrolysis of glucose 6-phosphate, resulting in the creation of a phosphate group and free glucose:
: D-glucose 6-phos ...
and released into the blood. Glucagon and epinephrine also stimulate gluconeogenesis, which coverts non-carbohydrate substrates into G6P, which joins the G6P derived from glycogen, or substitutes for it when the liver glycogen store have been depleted. This is critical for brain function, since the brain utilizes glucose as an energy source under most conditions.
The simultaneously phosphorylation of, particularly,
phosphofructokinase
Phosphofructokinase (PFK) is a kinase enzyme that phosphorylates fructose 6-phosphate in glycolysis.
Function
The enzyme-catalysed transfer of a phosphoryl group from ATP is an important reaction in a wide variety of biological processes. ...
, but also, to a certain extent pyruvate kinase, prevents glycolysis occurring at the same time as gluconeogenesis and glycogenolysis.
Hexokinase and glucokinase
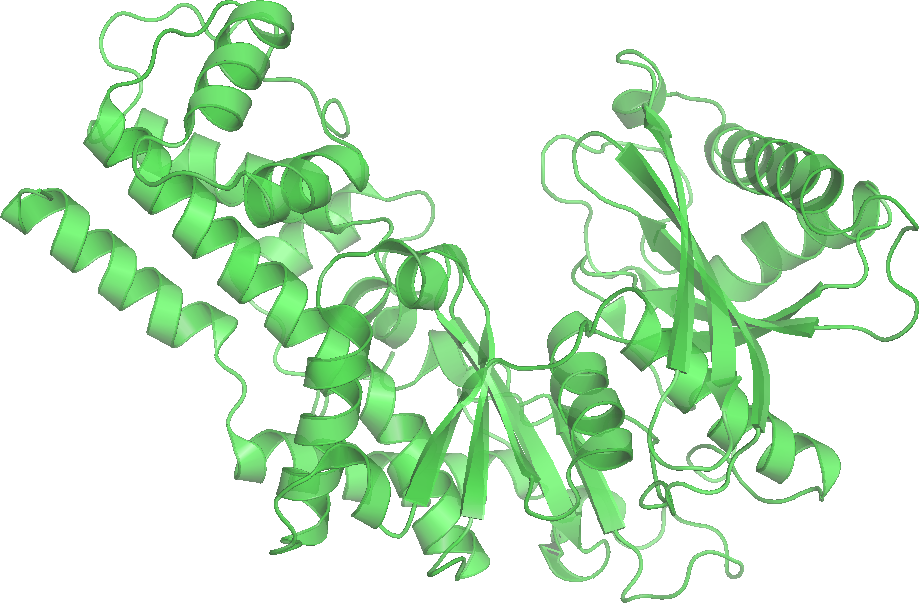
All cells contain the enzyme
hexokinase
A hexokinase is an enzyme that phosphorylates hexoses (six-carbon sugars), forming hexose phosphate. In most organisms, glucose is the most important substrate for hexokinases, and glucose-6-phosphate is the most important product. Hexok ...
, which catalyzes the conversion of glucose that has entered the cell into
glucose-6-phosphate
Glucose 6-phosphate (G6P, sometimes called the Robison ester) is a glucose sugar phosphorylated at the hydroxy group on carbon 6. This dianion is very common in cells as the majority of glucose entering a cell will become phosphorylated in this wa ...
(G6P). Since the cell membrane is impervious to G6P, hexokinase essentially acts to transport glucose into the cells from which it can then no longer escape. Hexokinase is inhibited by high levels of G6P in the cell. Thus the rate of entry of glucose into cells partially depends on how fast G6P can be disposed of by glycolysis, and by
glycogen synthesis (in the cells which store glycogen, namely liver and muscles).
Glucokinase
Glucokinase () is an enzyme that facilitates phosphorylation of glucose to glucose-6-phosphate. Glucokinase occurs in cells in the liver and pancreas of humans and most other vertebrates. In each of these organs it plays an important role i ...
, unlike
hexokinase
A hexokinase is an enzyme that phosphorylates hexoses (six-carbon sugars), forming hexose phosphate. In most organisms, glucose is the most important substrate for hexokinases, and glucose-6-phosphate is the most important product. Hexok ...
, is not inhibited by G6P. It occurs in liver cells, and will only phosphorylate the glucose entering the cell to form
glucose-6-phosphate
Glucose 6-phosphate (G6P, sometimes called the Robison ester) is a glucose sugar phosphorylated at the hydroxy group on carbon 6. This dianion is very common in cells as the majority of glucose entering a cell will become phosphorylated in this wa ...
(G6P), when the glucose in the blood is abundant. This being the first step in the glycolytic pathway in the liver, it therefore imparts an additional layer of control of the glycolytic pathway in this organ.
Phosphofructokinase

Phosphofructokinase
Phosphofructokinase (PFK) is a kinase enzyme that phosphorylates fructose 6-phosphate in glycolysis.
Function
The enzyme-catalysed transfer of a phosphoryl group from ATP is an important reaction in a wide variety of biological processes. ...
is an important control point in the glycolytic pathway, since it is one of the irreversible steps and has key allosteric effectors,
AMP #REDIRECT Amp
{{Redirect category shell, {{R from other capitalisation{{R from ambiguous page ...
and
fructose 2,6-bisphosphate
Fructose 2,6-bisphosphate, abbreviated Fru-2,6-''P''2, is a metabolite that allosterically affects the activity of the enzymes phosphofructokinase 1 (PFK-1) and fructose 1,6-bisphosphatase (FBPase-1) to regulate glycolysis and gluconeogenesis. F ...
(F2,6BP).
Fructose 2,6-bisphosphate
Fructose 2,6-bisphosphate, abbreviated Fru-2,6-''P''2, is a metabolite that allosterically affects the activity of the enzymes phosphofructokinase 1 (PFK-1) and fructose 1,6-bisphosphatase (FBPase-1) to regulate glycolysis and gluconeogenesis. F ...
(F2,6BP) is a very potent activator of phosphofructokinase (PFK-1) that is synthesized when F6P is phosphorylated by a second phosphofructokinase (
PFK2). In the liver, when blood sugar is low and
glucagon elevates cAMP,
PFK2 is phosphorylated by
protein kinase A
In cell biology, protein kinase A (PKA) is a family of enzymes whose activity is dependent on cellular levels of cyclic AMP (cAMP). PKA is also known as cAMP-dependent protein kinase (). PKA has several functions in the cell, including regulatio ...
. The phosphorylation inactivates
PFK2, and another domain on this protein becomes active as
fructose bisphosphatase-2, which converts F2,6BP back to F6P. Both
glucagon and
epinephrine cause high levels of cAMP in the liver. The result of lower levels of liver fructose-2,6-bisphosphate is a decrease in activity of
phosphofructokinase
Phosphofructokinase (PFK) is a kinase enzyme that phosphorylates fructose 6-phosphate in glycolysis.
Function
The enzyme-catalysed transfer of a phosphoryl group from ATP is an important reaction in a wide variety of biological processes. ...
and an increase in activity of
fructose 1,6-bisphosphatase
The enzyme fructose bisphosphatase (EC 3.1.3.11; systematic name D-fructose-1,6-bisphosphate 1-phosphohydrolase) catalyses the conversion of fructose-1,6-bisphosphate to fructose 6-phosphate in gluconeogenesis and the Calvin cycle, which ar ...
, so that gluconeogenesis (in essence, "glycolysis in reverse") is favored. This is consistent with the role of the liver in such situations, since the response of the liver to these hormones is to release glucose to the blood.
ATP competes with
AMP #REDIRECT Amp
{{Redirect category shell, {{R from other capitalisation{{R from ambiguous page ...
for the allosteric effector site on the PFK enzyme. ATP concentrations in cells are much higher than those of AMP, typically 100-fold higher, but the concentration of ATP does not change more than about 10% under physiological conditions, whereas a 10% drop in ATP results in a 6-fold increase in AMP. Thus, the relevance of ATP as an allosteric effector is questionable. An increase in AMP is a consequence of a decrease in
energy charge
The adenylate energy charge is an index used to measure the energy status of biological cells.
ATP or Mg-ATP is the principal molecule for storing and transferring energy in the cell : it is used for biosynthetic pathways, maintenance of transmem ...
in the cell.
Citrate
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the ...
inhibits phosphofructokinase when tested ''in vitro'' by enhancing the inhibitory effect of ATP. However, it is doubtful that this is a meaningful effect ''in vivo'', because citrate in the cytosol is utilized mainly for conversion to
acetyl-CoA for
fatty acid
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, ...
and
cholesterol
Cholesterol is any of a class of certain organic molecules called lipids. It is a sterol (or modified steroid), a type of lipid. Cholesterol is biosynthesized by all animal cells and is an essential structural component of animal cell mem ...
synthesis.
TIGAR
Tigar may refer to:
People
* Edward Wharton-Tigar (c. 1913–1995), British mining executive, World War II spy and saboteur
* Kenneth Tigar (born 1942), American actor
* Jon S. Tigar (born 1962), United States District Judge, son of Michael Tigar ...
, a p53 induced enzyme, is responsible for the regulation of
phosphofructokinase
Phosphofructokinase (PFK) is a kinase enzyme that phosphorylates fructose 6-phosphate in glycolysis.
Function
The enzyme-catalysed transfer of a phosphoryl group from ATP is an important reaction in a wide variety of biological processes. ...
and acts to protect against oxidative stress. TIGAR is a single enzyme with dual function that regulates F2,6BP. It can behave as a phosphatase (fructuose-2,6-bisphosphatase) which cleaves the phosphate at carbon-2 producing F6P. It can also behave as a kinase (PFK2) adding a phosphate onto carbon-2 of F6P which produces F2,6BP. In humans, the TIGAR protein is encoded by ''C12orf5'' gene. The TIGAR enzyme will hinder the forward progression of glycolysis, by creating a build up of fructose-6-phosphate (F6P) which is isomerized into glucose-6-phosphate (G6P). The accumulation of G6P will shunt carbons into the pentose phosphate pathway.
Pyruvate kinase

The final step of glycolysis is catalysed by pyruvate kinase to form pyruvate and another ATP. It is regulated by a range of different transcriptional, covalent and non-covalent regulation mechanisms, which can vary widely in different tissues. For example, in the liver, pyruvate kinase is regulated based on glucose availability. During fasting (no glucose available),
glucagon activates
protein kinase A
In cell biology, protein kinase A (PKA) is a family of enzymes whose activity is dependent on cellular levels of cyclic AMP (cAMP). PKA is also known as cAMP-dependent protein kinase (). PKA has several functions in the cell, including regulatio ...
which phosphorylates pyruvate kinase to inhibit it.
An increase in blood sugar leads to secretion of
insulin, which activates
protein phosphatase 1
Protein phosphatase 1 (PP1) belongs to a certain class of phosphatases known as protein serine/threonine phosphatases. This type of phosphatase includes metal-dependent protein phosphatases (PPMs) and aspartate-based phosphatases. PP1 has been fo ...
, leading to dephosphorylation and re-activation of pyruvate kinase.
These controls prevent pyruvate kinase from being active at the same time as the enzymes that catalyze the reverse reaction (
pyruvate carboxylase
Pyruvate carboxylase (PC) encoded by the gene PC is an enzyme () of the ligase class that catalyzes (depending on the species) the physiologically irreversible carboxylation of pyruvate to form oxaloacetate (OAA).
Image:Pyruvic-acid-2D-sk ...
and
phosphoenolpyruvate carboxykinase
Phosphoenolpyruvate carboxykinase (, PEPCK) is an enzyme in the lyase family used in the metabolic pathway of gluconeogenesis. It converts oxaloacetate into phosphoenolpyruvate and carbon dioxide.
It is found in two forms, cytosolic and mitoch ...
), preventing a
futile cycle
A futile cycle, also known as a substrate cycle, occurs when two metabolic pathways run simultaneously in opposite directions and have no overall effect other than to dissipate energy in the form of heat. The reason this cycle was called "futile" c ...
.
Conversely, the isoform of pyruvate kinasein found in muscle is not affected by
protein kinase A
In cell biology, protein kinase A (PKA) is a family of enzymes whose activity is dependent on cellular levels of cyclic AMP (cAMP). PKA is also known as cAMP-dependent protein kinase (). PKA has several functions in the cell, including regulatio ...
(which is activated by adrenaline in that tissue), so that glycolysis remains active in muscles even during fasting.
Post-glycolysis processes
The overall process of glycolysis is:
:Glucose + 2 NAD
+ + 2 ADP + 2 P
i → 2 pyruvate + 2 NADH + 2 H
+ + 2 ATP
If glycolysis were to continue indefinitely, all of the NAD
+ would be used up, and glycolysis would stop. To allow glycolysis to continue, organisms must be able to oxidize NADH back to NAD
+. How this is performed depends on which external electron acceptor is available.
Anoxic regeneration of NAD+
One method of doing this is to simply have the pyruvate do the oxidation; in this process, pyruvate is converted to
lactate (the
conjugate base
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid donates a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a ...
of lactic acid) in a process called
lactic acid fermentation
Lactic acid fermentation is a metabolic process by which glucose or other six-carbon sugars (also, disaccharides of six-carbon sugars, e.g. sucrose or lactose) are converted into cellular energy and the metabolite lactate, which is lactic acid i ...
:
:Pyruvate + NADH + H
+ → lactate + NAD
+
This process occurs in the
bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of prokaryotic microorganisms. Typically a few micrometr ...
involved in making
yogurt
Yogurt (; , from tr, yoğurt, also spelled yoghurt, yogourt or yoghourt) is a food produced by bacterial fermentation of milk. The bacteria used to make yogurt are known as ''yogurt cultures''. Fermentation of sugars in the milk by these bac ...
(the lactic acid causes the milk to curdle). This process also occurs in animals under hypoxic (or partially anaerobic) conditions, found, for example, in overworked muscles that are starved of oxygen. In many tissues, this is a cellular last resort for energy; most animal tissue cannot tolerate anaerobic conditions for an extended period of time.
Some organisms, such as yeast, convert NADH back to NAD
+ in a process called
ethanol fermentation
Ethanol fermentation, also called alcoholic fermentation, is a biological process which converts sugars such as glucose, fructose, and sucrose into cellular energy, producing ethanol and carbon dioxide as by-products. Because yeasts perform this ...
. In this process, the pyruvate is converted first to acetaldehyde and carbon dioxide, and then to ethanol.
Lactic acid fermentation
Lactic acid fermentation is a metabolic process by which glucose or other six-carbon sugars (also, disaccharides of six-carbon sugars, e.g. sucrose or lactose) are converted into cellular energy and the metabolite lactate, which is lactic acid i ...
and
ethanol fermentation
Ethanol fermentation, also called alcoholic fermentation, is a biological process which converts sugars such as glucose, fructose, and sucrose into cellular energy, producing ethanol and carbon dioxide as by-products. Because yeasts perform this ...
can occur in the absence of oxygen. This anaerobic fermentation allows many single-cell organisms to use glycolysis as their only energy source.
Anoxic regeneration of NAD
+ is only an effective means of energy production during short, intense exercise in vertebrates, for a period ranging from 10 seconds to 2 minutes during a maximal effort in humans. (At lower exercise intensities it can sustain muscle activity in
diving animals, such as seals, whales and other aquatic vertebrates, for very much longer periods of time.) Under these conditions NAD
+ is replenished by NADH donating its electrons to pyruvate to form lactate. This produces 2 ATP molecules per glucose molecule, or about 5% of glucose's energy potential (38 ATP molecules in bacteria). But the speed at which ATP is produced in this manner is about 100 times that of oxidative phosphorylation. The pH in the cytoplasm quickly drops when hydrogen ions accumulate in the muscle, eventually inhibiting the enzymes involved in glycolysis.
The burning sensation in muscles during hard exercise can be attributed to the release of hydrogen ions during the shift to glucose fermentation from glucose oxidation to carbon dioxide and water, when aerobic metabolism can no longer keep pace with the energy demands of the muscles. These hydrogen ions form a part of lactic acid. The body falls back on this less efficient but faster method of producing ATP under low oxygen conditions. This is thought to have been the primary means of energy production in earlier organisms before oxygen reached high concentrations in the atmosphere between 2000 and 2500 million years ago, and thus would represent a more ancient form of energy production than the aerobic replenishment of NAD
+ in cells.
The liver in mammals gets rid of this excess lactate by transforming it back into pyruvate under aerobic conditions; see
Cori cycle
The Cori cycle (also known as the lactic acid cycle), named after its discoverers, Carl Ferdinand Cori and Gerty Cori, is a metabolic pathway in which lactate, produced by anaerobic glycolysis in muscles, is transported to the liver and convert ...
.
Fermentation of pyruvate to lactate is sometimes also called "anaerobic glycolysis", however, glycolysis ends with the production of pyruvate regardless of the presence or absence of oxygen.
In the above two examples of fermentation, NADH is oxidized by transferring two electrons to pyruvate. However, anaerobic bacteria use a wide variety of compounds as the terminal electron acceptors in
cellular respiration
Cellular respiration is the process by which biological fuels are oxidised in the presence of an inorganic electron acceptor such as oxygen to produce large amounts of energy, to drive the bulk production of ATP. Cellular respiration may be des ...
: nitrogenous compounds, such as nitrates and nitrites; sulfur compounds, such as sulfates, sulfites, sulfur dioxide, and elemental sulfur; carbon dioxide; iron compounds; manganese compounds; cobalt compounds; and uranium compounds.
Aerobic regeneration of NAD+, and disposal of pyruvate
In
aerobic
Aerobic means "requiring air," in which "air" usually means oxygen.
Aerobic may also refer to
* Aerobic exercise, prolonged exercise of moderate intensity
* Aerobics, a form of aerobic exercise
* Aerobic respiration, the aerobic process of cel ...
eukaryotes, a complex mechanism has developed to use the oxygen in air as the final electron acceptor.
Aerobic
Aerobic means "requiring air," in which "air" usually means oxygen.
Aerobic may also refer to
* Aerobic exercise, prolonged exercise of moderate intensity
* Aerobics, a form of aerobic exercise
* Aerobic respiration, the aerobic process of cel ...
prokaryotes, which lack mitochondria, use a variety of
simpler mechanisms.
* Firstly, the
NADH + H+ generated by glycolysis has to be transferred to the mitochondrion to be oxidized, and thus to regenerate the NAD
+ necessary for glycolysis to continue. However the inner mitochondrial membrane is impermeable to NADH and NAD
+.
Use is therefore made of two “shuttles” to transport the electrons from NADH across the mitochondrial membrane. They are the
malate-aspartate shuttle
The malate-aspartate shuttle (sometimes simply the malate shuttle) is a biochemical system for translocating electrons produced during glycolysis across the semipermeable inner membrane of the mitochondrion for oxidative phosphorylation in eukaryo ...
and the
glycerol phosphate shuttle
The glycerol-3-phosphate shuttle is a mechanism that regenerates NAD+ from NADH, a by-product of glycolysis. The shuttle consists of the sequential activity of two proteins: GPD1 which transfers an electron pair from NADH to dihydroxyacetone phosp ...
. In the former the electrons from NADH are transferred to cytosolic
oxaloacetate
Oxaloacetic acid (also known as oxalacetic acid or OAA) is a crystalline organic compound with the chemical formula HO2CC(O)CH2CO2H. Oxaloacetic acid, in the form of its conjugate base oxaloacetate, is a metabolic intermediate in many processes ...
to form
malate
Malic acid is an organic compound with the molecular formula . It is a dicarboxylic acid that is made by all living organisms, contributes to the sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms (L ...
. The malate then traverses the inner mitochondrial membrane into the mitochondrial matrix, where it is reoxidized by NAD
+ forming intra-mitochondrial oxaloacetate and NADH. The oxaloacetate is then re-cycled to the cytosol via its conversion to aspartate which is readily transported out of the mitochondrion. In the glycerol phosphate shuttle electrons from cytosolic NADH are transferred to
dihydroxyacetone
Dihydroxyacetone (; DHA), also known as glycerone, is a simple saccharide (a triose) with formula .
DHA is primarily used as an ingredient in sunless tanning products. It is often derived from plant sources such as sugar beets and sugar cane, an ...
to form
glycerol-3-phosphate
''sn''-Glycerol 3-phosphate is the organic ion with the formula HOCH2CH(OH)CH2OPO32-. It is one of three stereoisomers of the ester of dibasic phosphoric acid (HOPO32-) and glycerol. It is a component of glycerophospholipids. Equally appropria ...
which readily traverses the outer mitochondrial membrane. Glycerol-3-phosphate is then reoxidized to dihydroxyacetone, donating its electrons to
FAD
A fad or trend is any form of collective behavior that develops within a culture, a generation or social group in which a group of people enthusiastically follow an impulse for a short period.
Fads are objects or behaviors that achieve short- ...
instead of NAD
+.
This reaction takes place on the inner mitochondrial membrane, allowing FADH
2 to donate its electrons directly to coenzyme Q (
ubiquinone
Coenzyme Q, also known as ubiquinone and marketed as CoQ10, is a coenzyme family that is ubiquitous in animals and most bacteria (hence the name ubiquinone). In humans, the most common form is coenzyme Q10 or ubiquinone-10.
It is a 1,4-benzoq ...
) which is part of the
electron transport chain which ultimately transfers electrons to molecular oxygen , with the formation of water, and the release of energy eventually captured in the form of
ATP.
* The glycolytic end-product, pyruvate (plus NAD
+) is converted to
acetyl-CoA, and NADH + H
+ within the
mitochondria in a process called
pyruvate decarboxylation
Pyruvate decarboxylation or pyruvate oxidation, also known as the link reaction (or oxidative decarboxylation of pyruvate), is the conversion of pyruvate into acetyl-CoA by the enzyme complex pyruvate dehydrogenase complex.
The reaction may be s ...
.
* The resulting acetyl-CoA enters the
citric acid cycle
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and protein ...
(or Krebs Cycle), where the acetyl group of the acetyl-CoA is converted into carbon dioxide by two decarboxylation reactions with the formation of yet more intra-mitochondrial NADH + H
+.
* The intra-mitochondrial NADH + H
+ is oxidized to NAD
+ by the
electron transport chain, using oxygen as the final electron acceptor to form water. The energy released during this process is used to create a hydrogen ion (or proton) gradient across the inner membrane of the mitochondrion.
* Finally, the proton gradient is used to produce about 2.5
ATP for every NADH + H
+ oxidized in a process called
oxidative phosphorylation.
Conversion of carbohydrates into fatty acids and cholesterol
The pyruvate produced by glycolysis is an important intermediary in the conversion of carbohydrates into
fatty acids
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated and unsaturated compounds#Organic chemistry, saturated or unsaturated. Most naturally occurring fatty acids have an B ...
and
cholesterol
Cholesterol is any of a class of certain organic molecules called lipids. It is a sterol (or modified steroid), a type of lipid. Cholesterol is biosynthesized by all animal cells and is an essential structural component of animal cell mem ...
.
This occurs via the conversion of pyruvate into
acetyl-CoA in the
mitochondrion. However, this acetyl CoA needs to be transported into cytosol where the synthesis of fatty acids and cholesterol occurs. This cannot occur directly. To obtain cytosolic acetyl-CoA,
citrate
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the ...
(produced by the condensation of acetyl CoA with
oxaloacetate
Oxaloacetic acid (also known as oxalacetic acid or OAA) is a crystalline organic compound with the chemical formula HO2CC(O)CH2CO2H. Oxaloacetic acid, in the form of its conjugate base oxaloacetate, is a metabolic intermediate in many processes ...
) is removed from the
citric acid cycle
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and protein ...
and carried across the inner mitochondrial membrane into the
cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells ( intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondri ...
.
There it is cleaved by
ATP citrate lyase
ATP citrate synthase (also ATP citrate lyase (ACLY)) is an enzyme that in animals represents an important step in fatty acid biosynthesis. By converting citrate to acetyl-CoA, the enzyme links carbohydrate metabolism, which yields citrate as an ...
into acetyl-CoA and oxaloacetate. The oxaloacetate is returned to mitochondrion as malate (and then back into oxaloacetate to transfer more acetyl-CoA out of the mitochondrion). The cytosolic acetyl-CoA can be carboxylated by
acetyl-CoA carboxylase into
malonyl CoA
Malonyl-CoA is a coenzyme A derivative of malonic acid.
Functions
It plays a key role in chain elongation in fatty acid biosynthesis and polyketide biosynthesis.
Fatty acid biosynthesis
Malonyl-CoA provides 2-carbon units to fatty acids and commi ...
, the first committed step in the
synthesis of fatty acids, or it can be combined with
acetoacetyl-CoA
Acetoacetyl CoA is the precursor of HMG-CoA in the mevalonate pathway, which is essential for cholesterol biosynthesis. It also takes a similar role in the ketone bodies synthesis (ketogenesis) pathway of the liver. In the ketone bodies digestion ...
to form 3-hydroxy-3-methylglutaryl-CoA (
HMG-CoA
β-Hydroxy β-methylglutaryl-CoA (HMG-CoA), also known as 3-hydroxy-3-methylglutaryl coenzyme A, is an intermediate in the mevalonate and ketogenesis pathways. It is formed from acetyl CoA and acetoacetyl CoA by HMG-CoA synthase. The research of ...
) which is the rate limiting step controlling the
synthesis of cholesterol.
Cholesterol can be used as is, as a structural component of cellular membranes, or it can be used to synthesize the
steroid hormones
A steroid hormone is a steroid that acts as a hormone. Steroid hormones can be grouped into two classes: corticosteroids (typically made in the adrenal cortex, hence ''cortico-'') and sex steroids (typically made in the gonads or placenta). Withi ...
,
bile salts
Bile acids are steroid acids found predominantly in the bile of mammals and other vertebrates. Diverse bile acids are synthesized in the liver. Bile acids are conjugated with taurine or glycine residues to give anions called bile salts.
Primary b ...
, and
vitamin D
Vitamin D is a group of fat-soluble secosteroids responsible for increasing intestinal absorption of calcium, magnesium, and phosphate, and many other biological effects. In humans, the most important compounds in this group are vitamin D3 (c ...
.
Conversion of pyruvate into oxaloacetate for the citric acid cycle
Pyruvate molecules produced by glycolysis are
actively transported across the inner
mitochondrial membrane, and into the matrix where they can either be
oxidized
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
and combined with
coenzyme A to form , acetyl-CoA, and NADH,
or they can be
carboxylated
Carboxylation is a chemical reaction in which a carboxylic acid is produced by treating a substrate with carbon dioxide. The opposite reaction is decarboxylation. In chemistry, the term carbonation is sometimes used synonymously with carboxylat ...
(by
pyruvate carboxylase
Pyruvate carboxylase (PC) encoded by the gene PC is an enzyme () of the ligase class that catalyzes (depending on the species) the physiologically irreversible carboxylation of pyruvate to form oxaloacetate (OAA).
Image:Pyruvic-acid-2D-sk ...
) to form
oxaloacetate
Oxaloacetic acid (also known as oxalacetic acid or OAA) is a crystalline organic compound with the chemical formula HO2CC(O)CH2CO2H. Oxaloacetic acid, in the form of its conjugate base oxaloacetate, is a metabolic intermediate in many processes ...
. This latter reaction "fills up" the amount of oxaloacetate in the citric acid cycle, and is therefore an
anaplerotic reaction (from the Greek meaning to "fill up"), increasing the cycle's capacity to metabolize acetyl-CoA when the tissue's energy needs (e.g. in
heart
The heart is a muscular organ in most animals. This organ pumps blood through the blood vessels of the circulatory system. The pumped blood carries oxygen and nutrients to the body, while carrying metabolic waste such as carbon dioxide to t ...
and
skeletal muscle) are suddenly increased by activity.
In the
citric acid cycle
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and protein ...
all the intermediates (e.g. citrate, iso-citrate, alpha-ketoglutarate, succinate, fumarate, malate and oxaloacetate) are regenerated during each turn of the cycle. Adding more of any of these intermediates to the mitochondrion therefore means that that additional amount is retained within the cycle, increasing all the other intermediates as one is converted into the other. Hence the addition of oxaloacetate greatly increases the amounts of all the citric acid intermediates, thereby increasing the cycle's capacity to metabolize acetyl CoA, converting its acetate component into and water, with the release of enough energy to form 11
ATP and 1
GTP molecule for each additional molecule of acetyl CoA that combines with oxaloacetate in the cycle.
To cataplerotically remove oxaloacetate from the citric cycle,
malate
Malic acid is an organic compound with the molecular formula . It is a dicarboxylic acid that is made by all living organisms, contributes to the sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms (L ...
can be transported from the mitochondrion into the cytoplasm, decreasing the amount of oxaloacetate that can be regenerated.
Furthermore, citric acid intermediates are
constantly used to form a variety of substances such as the purines, pyrimidines and porphyrins.
Intermediates for other pathways
This article concentrates on the
catabolic
Catabolism () is the set of metabolic pathways that breaks down molecules into smaller units that are either oxidized to release energy or used in other anabolic reactions. Catabolism breaks down large molecules (such as polysaccharides, lipid ...
role of glycolysis with regard to converting potential chemical energy to usable chemical energy during the oxidation of glucose to pyruvate. Many of the metabolites in the glycolytic pathway are also used by
anabolic
Anabolism () is the set of metabolic pathways that construct molecules from smaller units. These reactions require energy, known also as an endergonic process. Anabolism is the building-up aspect of metabolism, whereas catabolism is the breaking ...
pathways, and, as a consequence, flux through the pathway is critical to maintain a supply of carbon skeletons for biosynthesis.
The following metabolic pathways are all strongly reliant on glycolysis as a source of metabolites: and many more.
*
Pentose phosphate pathway
The pentose phosphate pathway (also called the phosphogluconate pathway and the hexose monophosphate shunt and the HMP Shunt) is a metabolic pathway parallel to glycolysis. It generates NADPH and pentoses (5-carbon sugars) as well as ribose 5-pho ...
, which begins with the dehydrogenation of
glucose-6-phosphate
Glucose 6-phosphate (G6P, sometimes called the Robison ester) is a glucose sugar phosphorylated at the hydroxy group on carbon 6. This dianion is very common in cells as the majority of glucose entering a cell will become phosphorylated in this wa ...
, the first intermediate to be produced by glycolysis, produces various pentose sugars, and
NADPH for the synthesis of
fatty acid
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, ...
s and
cholesterol
Cholesterol is any of a class of certain organic molecules called lipids. It is a sterol (or modified steroid), a type of lipid. Cholesterol is biosynthesized by all animal cells and is an essential structural component of animal cell mem ...
.
*
Glycogen synthesis also starts with glucose-6-phosphate at the beginning of the glycolytic pathway.
*
Glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known ...
, for the formation of
triglyceride
A triglyceride (TG, triacylglycerol, TAG, or triacylglyceride) is an ester derived from glycerol and three fatty acids (from ''wikt:tri-#Prefix, tri-'' and ''glyceride'').
Triglycerides are the main constituents of body fat in humans and other ...
s and
phospholipids, is produced from the glycolytic intermediate
glyceraldehyde-3-phosphate
Glyceraldehyde 3-phosphate, also known as triose phosphate or 3-phosphoglyceraldehyde and abbreviated as G3P, GA3P, GADP, GAP, TP, GALP or PGAL, is a metabolite that occurs as an intermediate in several central pathways of all organisms.Nelson, ...
.
* Various post-glycolytic pathways:
:*
Fatty acid synthesis
In biochemistry, fatty acid synthesis is the creation of fatty acids from acetyl-CoA and NADPH through the action of enzymes called fatty acid synthases. This process takes place in the cytoplasm of the cell. Most of the acetyl-CoA which is co ...
:*
Cholesterol synthesis
The mevalonate pathway, also known as the isoprenoid pathway or HMG-CoA reductase pathway is an essential metabolic pathway present in eukaryotes, archaea, and some bacteria. The pathway produces two five-carbon building blocks called isopenten ...
:* The
citric acid cycle
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and protein ...
which in turn leads to:
::*
Amino acid synthesis
Amino acid synthesis is the set of biochemical processes (metabolic pathways) by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to s ...
::*
Nucleotide synthesis
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules with ...
::*
Tetrapyrrole synthesis
Although
gluconeogenesis and glycolysis share many intermediates the one is not functionally a branch or tributary of the other. There are two regulatory steps in both pathways which, when active in the one pathway, are automatically inactive in the other. The two processes can therefore not be simultaneously active.
Indeed, if both sets of reactions were highly active at the same time the net result would be the hydrolysis of four high energy phosphate bonds (two ATP and two GTP) per reaction cycle.
is the oxidizing agent in glycolysis, as it is in most other energy yielding metabolic reactions (e.g.
beta-oxidation of fatty acids, and during the
citric acid cycle
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and protein ...
). The NADH thus produced is primarily used to ultimately transfer electrons to to produce water, or, when is not available, to produced compounds such as
lactate or
ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a ...
(see ''Anoxic regeneration of NAD
+'' above). NADH is rarely used for synthetic processes, the notable exception being
gluconeogenesis. During
fatty acid
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, ...
and
cholesterol synthesis
The mevalonate pathway, also known as the isoprenoid pathway or HMG-CoA reductase pathway is an essential metabolic pathway present in eukaryotes, archaea, and some bacteria. The pathway produces two five-carbon building blocks called isopenten ...
the reducing agent is
NADPH. This difference exemplifies a general principle that NADPH is consumed during biosynthetic reactions, whereas NADH is generated in energy-yielding reactions.
The source of the NADPH is two-fold. When
malate
Malic acid is an organic compound with the molecular formula . It is a dicarboxylic acid that is made by all living organisms, contributes to the sour taste of fruits, and is used as a food additive. Malic acid has two stereoisomeric forms (L ...
is oxidatively decarboxylated by “NADP
+-linked malic enzyme"
pyruvate, and NADPH are formed. NADPH is also formed by the
pentose phosphate pathway
The pentose phosphate pathway (also called the phosphogluconate pathway and the hexose monophosphate shunt and the HMP Shunt) is a metabolic pathway parallel to glycolysis. It generates NADPH and pentoses (5-carbon sugars) as well as ribose 5-pho ...
which converts glucose into ribose, which can be used in synthesis of
nucleotides
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules with ...
and
nucleic acids
Nucleic acids are biopolymers, macromolecules, essential to all known forms of life. They are composed of nucleotides, which are the monomers made of three components: a 5-carbon sugar, a phosphate group and a nitrogenous base. The two main cl ...
, or it can be catabolized to pyruvate.
Glycolysis in disease
Diabetes
Cellular uptake of glucose occurs in response to insulin signals, and glucose is subsequently broken down through glycolysis, lowering blood sugar levels. However, the low insulin levels seen in diabetes result in hyperglycemia, where glucose levels in the blood rise and glucose is not properly taken up by cells. Hepatocytes further contribute to this hyperglycemia through
gluconeogenesis. Glycolysis in hepatocytes controls hepatic glucose production, and when glucose is overproduced by the liver without having a means of being broken down by the body, hyperglycemia results.
Genetic diseases
Glycolytic mutations are generally rare due to importance of the metabolic pathway, this means that the majority of occurring mutations result in an inability for the cell to respire, and therefore cause the death of the cell at an early stage. However, some mutations are seen with one notable example being
Pyruvate kinase deficiency
Pyruvate kinase deficiency is an inherited metabolic disorder of the enzyme pyruvate kinase which affects the survival of red blood cells. Both autosomal dominant and recessive inheritance have been observed with the disorder; classically, and ...
, leading to chronic hemolytic anemia.
Cancer
Malignant tumor cells perform glycolysis at a rate that is ten times faster than their noncancerous tissue counterparts. During their genesis, limited capillary support often results in hypoxia (decreased O2 supply) within the tumor cells. Thus, these cells rely on anaerobic metabolic processes such as glycolysis for ATP (adenosine triphosphate). Some tumor cells overexpress specific glycolytic enzymes which result in higher rates of glycolysis. Often these enzymes are Isoenzymes, of traditional glycolysis enzymes, that vary in their susceptibility to traditional feedback inhibition. The increase in glycolytic activity ultimately counteracts the effects of hypoxia by generating sufficient ATP from this anaerobic pathway. This phenomenon was first described in 1930 by
Otto Warburg and is referred to as the
Warburg effect. The
Warburg hypothesis claims that cancer is primarily caused by dysfunctionality in mitochondrial metabolism, rather than because of the uncontrolled growth of cells.
A number of theories have been advanced to explain the Warburg effect. One such theory suggests that the increased glycolysis is a normal protective process of the body and that malignant change could be primarily caused by energy metabolism.
This high glycolysis rate has important medical applications, as high aerobic glycolysis by malignant tumors is utilized clinically to diagnose and monitor treatment responses of
cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
s by
imaging
Imaging is the representation or reproduction of an object's form; especially a visual representation (i.e., the formation of an image).
Imaging technology is the application of materials and methods to create, preserve, or duplicate images.
...
uptake of
2-18F-2-deoxyglucose (FDG) (a
radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi ...
modified hexokinase
substrate) with
positron emission tomography (PET).
There is ongoing research to affect mitochondrial metabolism and treat cancer by reducing glycolysis and thus starving cancerous cells in various new ways, including a
ketogenic diet
The ketogenic diet is a high- fat, adequate-protein, low-carbohydrate dietary therapy that in conventional medicine is used mainly to treat hard-to-control (refractory) epilepsy in children. The diet forces the body to burn fats rather than ca ...
.
Interactive pathway map
The diagram below shows human protein names. Names in other organisms may be different and the number of
isozyme In biochemistry, isozymes (also known as isoenzymes or more generally as multiple forms of enzymes) are enzymes that differ in amino acid sequence but catalyze the same chemical reaction. Isozymes usually have different kinetic parameters (e.g. dif ...
s (such as HK1, HK2, ...) is likely to be different too.
Alternative nomenclature
Some of the metabolites in glycolysis have alternative names and nomenclature. In part, this is because some of them are common to other pathways, such as the
Calvin cycle.
Structure of glycolysis components in Fischer projections and polygonal model
The intermediates of glycolysis depicted in Fischer projections show the chemical changing step by step. Such image can be compared to polygonal model representation.
Another comparation of Fischer projections and Poligonal Model in glycolysis is shown in a video. Video animations in the same channel in YouTube can be seen for another metabolic pathway (Krebs Cycle) and the representation and applying of Polygonal Model in Organic Chemistry
See also
*
Carbohydrate catabolism
*
Citric acid cycle
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and protein ...
*
Cori cycle
The Cori cycle (also known as the lactic acid cycle), named after its discoverers, Carl Ferdinand Cori and Gerty Cori, is a metabolic pathway in which lactate, produced by anaerobic glycolysis in muscles, is transported to the liver and convert ...
*
Fermentation (biochemistry)
Fermentation is a metabolic process that produces chemical changes in organic substrates through the action of enzymes. In biochemistry, it is narrowly defined as the extraction of energy from carbohydrates in the absence of oxygen. In food ...
*
Gluconeogenesis
*
Glycolytic oscillation In biochemistry, a glycolytic oscillation is the repetitive fluctuation of in the concentrations of metabolites, classically observed experimentally in yeast and muscle. The first observations of oscillatory behaviour in glycolysis were made by Duy ...
*
Pentose phosphate pathway
The pentose phosphate pathway (also called the phosphogluconate pathway and the hexose monophosphate shunt and the HMP Shunt) is a metabolic pathway parallel to glycolysis. It generates NADPH and pentoses (5-carbon sugars) as well as ribose 5-pho ...
*
Pyruvate decarboxylation
Pyruvate decarboxylation or pyruvate oxidation, also known as the link reaction (or oxidative decarboxylation of pyruvate), is the conversion of pyruvate into acetyl-CoA by the enzyme complex pyruvate dehydrogenase complex.
The reaction may be s ...
*
Triose kinase
In enzymology, a triokinase () is an enzyme that catalyzes the chemical reaction
:ATP + D-glyceraldehyde \rightleftharpoons ADP + D-glyceraldehyde 3-phosphate
Thus, the two substrates of this enzyme are ATP and D-glyceraldehyde, whereas its t ...
References
External links
A Detailed Glycolysis Animation providedby
IUBMB
The International Union of Biochemistry and Molecular Biology (IUBMB) is an international non-governmental organisation concerned with biochemistry and molecular biology. Formed in 1955 as the International Union of Biochemistry (IUB), the union ...
Adobe FlashRequired)
at RCSB PDB
Glycolytic cycle with animationsat wdv.com
Metabolism, Cellular Respiration and Photosynthesis - The Virtual Library of Biochemistry, Molecular Biology and Cell Biologyat ufp.pt
at
ExPASy
*
''metpath'': Interactive representation of glycolysis
{{Authority control
Biochemistry
Carbohydrates
Cellular respiration
Metabolic pathways
 Glycolysis is the
Glycolysis is the  Insight into the component steps of glycolysis were provided by the non-cellular fermentation experiments of
Insight into the component steps of glycolysis were provided by the non-cellular fermentation experiments of  In the 1920s
In the 1920s  All cells contain the enzyme
All cells contain the enzyme 
 The final step of glycolysis is catalysed by pyruvate kinase to form pyruvate and another ATP. It is regulated by a range of different transcriptional, covalent and non-covalent regulation mechanisms, which can vary widely in different tissues. For example, in the liver, pyruvate kinase is regulated based on glucose availability. During fasting (no glucose available), glucagon activates
The final step of glycolysis is catalysed by pyruvate kinase to form pyruvate and another ATP. It is regulated by a range of different transcriptional, covalent and non-covalent regulation mechanisms, which can vary widely in different tissues. For example, in the liver, pyruvate kinase is regulated based on glucose availability. During fasting (no glucose available), glucagon activates