|
Oxaloacetic Acid
Oxaloacetic acid (also known as oxalacetic acid or OAA) is a crystalline organic compound with the chemical formula HO2CC(O)CH2CO2H. Oxaloacetic acid, in the form of its conjugate base oxaloacetate, is a metabolic intermediate in many processes that occur in animals. It takes part in gluconeogenesis, the urea cycle, the glyoxylate cycle, amino acid synthesis, fatty acid synthesis and the citric acid cycle. Properties Oxaloacetic acid undergoes successive deprotonations to give the dianion: :HO2CC(O)CH2CO2H −O2CC(O)CH2CO2H + H+, pKa = 2.22 :−O2CC(O)CH2CO2H −O2CC(O)CH2CO2− + H+, pKa = 3.89 At high pH, the enolizable proton is ionized: :−O2CC(O)CH2CO2− −O2CC(O−)CHCO2− + H+, pKa = 13.03 The enol forms of oxaloacetic acid are particularly stable, so much so that the two tautomers have different melting points (152 °C for the ''cis'' isoform and 184 °C for the ''trans'' isoform). This reaction is catalyzed by the enzyme oxaloacetate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Liv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malate Dehydrogenase
Malate dehydrogenase () (MDH) is an enzyme that reversibly catalyzes the oxidation of malate to oxaloacetate using the reduction of NAD+ to NADH. This reaction is part of many metabolic pathways, including the citric acid cycle. Other malate dehydrogenases, which have other EC numbers and catalyze other reactions oxidizing malate, have qualified names like malate dehydrogenase (NADP+). Isozymes Several isozymes of malate dehydrogenase exist. There are two main isoforms in eukaryotic cells. One is found in the mitochondrial matrix, participating as a key enzyme in the citric acid cycle that catalyzes the oxidation of malate. The other is found in the cytoplasm, assisting the malate-aspartate shuttle with exchanging reducing equivalents so that malate can pass through the mitochondrial membrane to be transformed into oxaloacetate for further cellular processes. Humans and most other mammals express the following two malate dehydrogenases: Protein families The ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
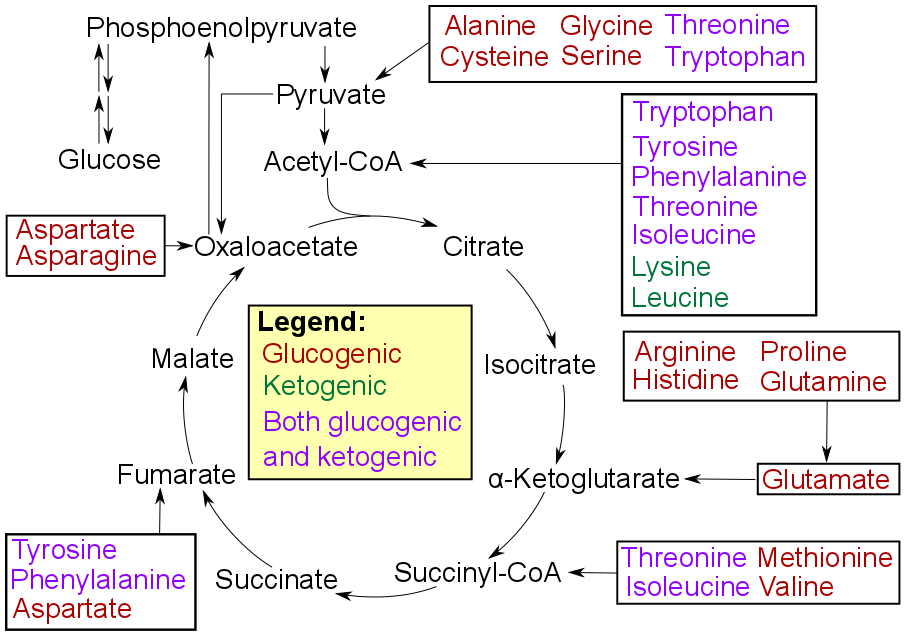
Gluconeogenesis
Gluconeogenesis (GNG) is a metabolic pathway that results in the generation of glucose from certain non- carbohydrate carbon substrates. It is a ubiquitous process, present in plants, animals, fungi, bacteria, and other microorganisms. In vertebrates, gluconeogenesis occurs mainly in the liver and, to a lesser extent, in the cortex of the kidneys. It is one of two primary mechanisms – the other being degradation of glycogen ( glycogenolysis) – used by humans and many other animals to maintain blood sugar levels, avoiding low levels ( hypoglycemia). In ruminants, because dietary carbohydrates tend to be metabolized by rumen organisms, gluconeogenesis occurs regardless of fasting, low-carbohydrate diets, exercise, etc. In many other animals, the process occurs during periods of fasting, starvation, low-carbohydrate diets, or intense exercise. In humans, substrates for gluconeogenesis may come from any non-carbohydrate sources that can be converted to pyruvate or in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Complex II
Succinate dehydrogenase (SDH) or succinate-coenzyme Q reductase (SQR) or respiratory complex II is an enzyme complex, found in many bacterial cells and in the inner mitochondrial membrane of eukaryotes. It is the only enzyme that participates in both the citric acid cycle and the electron transport chain. Histochemical analysis showing high succinate dehydrogenase in muscle demonstrates high mitochondrial content and high oxidative potential. In step 6 of the citric acid cycle, SQR catalyzes the oxidation of succinate to fumarate with the reduction of ubiquinone to ubiquinol. This occurs in the inner mitochondrial membrane by coupling the two reactions together. Structure Subunits Mitochondrial and many bacterial SQRs are composed of four structurally different subunits: two hydrophilic and two hydrophobic. The first two subunits, a flavoprotein (SdhA) and an iron-sulfur protein (SdhB), form a hydrophilic head where enzymatic activity of the complex takes place. SdhA c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citrate Synthase
The enzyme citrate synthase E.C. 2.3.3.1 (previously 4.1.3.7)] exists in nearly all living cells and stands as a pace-making enzyme in the first step of the citric acid cycle (or Krebs cycle). Citrate synthase is localized within eukaryotic cells in the mitochondrial matrix, but is encoded by nuclear DNA rather than mitochondrial. It is synthesized using cytoplasmic ribosomes, then transported into the mitochondrial matrix. Citrate synthase is commonly used as a quantitative enzyme marker for the presence of intact mitochondria. Maximal activity of citrate synthase indicates the mitochondrial content of skeletal muscle. The maximal activity can be increased by endurance training or high-intensity interval training, but maximal activity is further increased with high-intensity interval training. Citrate synthase catalyzes the condensation reaction of the two-carbon acetate residue from acetyl coenzyme A and a molecule of four-carbon oxaloacetate to form the six-carbon citra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citrate
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms. More than two million tons of citric acid are manufactured every year. It is used widely as an acidifier, as a flavoring, and a chelating agent. A citrate is a derivative of citric acid; that is, the salts, esters, and the polyatomic anion found in solution. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate. When part of a salt, the formula of the citrate anion is written as or . Natural occurrence and industrial production Citric acid occurs in a variety of fruits and vegetables, most notably citrus fruits. Lemons and limes have particularly high concentrations of the acid; it can constitute as much as 8% of the dry weight of these fruits (about 47 g/L in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetyl-CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized for energy production. Coenzyme A (CoASH or CoA) consists of a β-mercaptoethylamine group linked to the vitamin pantothenic acid (B5) through an amide linkage and 3'-phosphorylated ADP. The acetyl group (indicated in blue in the structural diagram on the right) of acetyl-CoA is linked to the sulfhydryl substituent of the β-mercaptoethylamine group. This thioester linkage is a "high energy" bond, which is particularly reactive. Hydrolysis of the thioester bond is exergonic (−31.5 kJ/mol). CoA is acetylated to acetyl-CoA by the breakdown of carbohydrates through glycolysis and by the breakdown of fatty acids through β-oxidation. Acetyl-CoA then enters the citric acid cycle, where the acetyl group is oxidized to carbon dioxide and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspartic Acid
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. Like all other amino acids, it contains an amino group and a carboxylic acid. Its α-amino group is in the protonated –NH form under physiological conditions, while its α-carboxylic acid group is deprotonated −COO− under physiological conditions. Aspartic acid has an acidic side chain (CH2COOH) which reacts with other amino acids, enzymes and proteins in the body. Under physiological conditions (pH 7.4) in proteins the side chain usually occurs as the negatively charged aspartate form, −COO−. It is a non-essential amino acid in humans, meaning the body can synthesize it as needed. It is encoded by the codons GAU and GAC. D-Aspartate is one of two D-amino acids commonly found in mammals. .html" ;"title="/sup>">/sup> In proteins aspartate sidechains are often hydrogen bonded to form asx turns or asx motifs, which frequently occur at th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transamination
Transamination is a chemical reaction that transfers an amino group to a ketoacid to form new amino acids. This pathway is responsible for the deamination of most amino acids. This is one of the major degradation pathways which convert essential amino acids to non-essential amino acids (amino acids that can be synthesized de novo by the organism). Transamination in biochemistry is accomplished by enzymes called transaminases or aminotransferases. α-ketoglutarate acts as the predominant amino-group acceptor and produces glutamate as the new amino acid. :Aminoacid + α-ketoglutarate ↔ α-keto acid + glutamate Glutamate's amino group, in turn, is transferred to oxaloacetate in a second transamination reaction yielding aspartate. :Glutamate + oxaloacetate ↔ α-ketoglutarate + aspartate Mechanism of action Transamination catalyzed by aminotransferase occurs in two stages. In the first step, the α amino group of an amino acid is transferred to the enzyme, producing t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoenolpyruvate Carboxylase
Phosphoenolpyruvate carboxylase (also known as PEP carboxylase, PEPCase, or PEPC; , PDB ID: 3ZGE) is an enzyme in the family of carboxy-lyases found in plants and some bacteria that catalyzes the addition of bicarbonate (HCO3−) to phosphoenolpyruvate (PEP) to form the four-carbon compound oxaloacetate and inorganic phosphate: :PEP + HCO3− → oxaloacetate + Pi This reaction is used for carbon fixation in CAM (crassulacean acid metabolism) and organisms, as well as to regulate flux through the citric acid cycle (also known as Krebs or TCA cycle) in bacteria and plants. The enzyme structure and its two step catalytic, irreversible mechanism have been well studied. PEP carboxylase is highly regulated, both by phosphorylation and allostery. Enzyme structure The PEP carboxylase enzyme is present in plants and some types of bacteria, but not in fungi or animals (including humans). The genes vary between organisms, but are strictly conserved around the active and allosteric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoenolpyruvate
Phosphoenolpyruvate (2-phosphoenolpyruvate, PEP) is the ester derived from the enol of pyruvate and phosphate. It exists as an anion. PEP is an important intermediate in biochemistry. It has the highest-energy phosphate bond found (−61.9 kJ/mol) in organisms, and is involved in glycolysis and gluconeogenesis. In plants, it is also involved in the biosynthesis of various aromatic compounds, and in carbon fixation; in bacteria, it is also used as the source of energy for the phosphotransferase system. In glycolysis PEP is formed by the action of the enzyme enolase on 2-phosphoglyceric acid. Metabolism of PEP to pyruvic acid by pyruvate kinase (PK) generates adenosine triphosphate (ATP) via substrate-level phosphorylation. ATP is one of the major currencies of chemical energy within cells. In gluconeogenesis PEP is formed from the decarboxylation of oxaloacetate and hydrolysis of one guanosine triphosphate molecule. This reaction is catalyzed by the enzyme phos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leaf
A leaf ( : leaves) is any of the principal appendages of a vascular plant stem, usually borne laterally aboveground and specialized for photosynthesis. Leaves are collectively called foliage, as in "autumn foliage", while the leaves, stem, flower, and fruit collectively form the shoot system. In most leaves, the primary photosynthetic tissue is the palisade mesophyll and is located on the upper side of the blade or lamina of the leaf but in some species, including the mature foliage of ''Eucalyptus'', palisade mesophyll is present on both sides and the leaves are said to be isobilateral. Most leaves are flattened and have distinct upper (adaxial) and lower ( abaxial) surfaces that differ in color, hairiness, the number of stomata (pores that intake and output gases), the amount and structure of epicuticular wax and other features. Leaves are mostly green in color due to the presence of a compound called chlorophyll that is essential for photosynthesis as it absorbs light ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.png)