Telomerase on:
[Wikipedia]
[Google]
[Amazon]
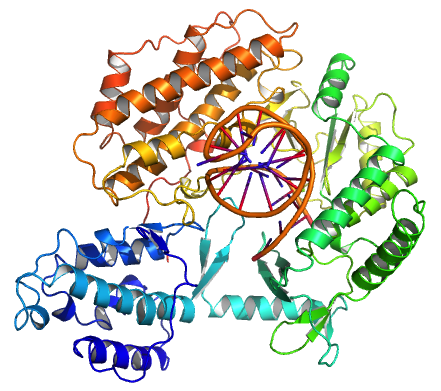 Telomerase, also called terminal transferase, is a
Telomerase, also called terminal transferase, is a
 The protein consists of four conserved domains (RNA-Binding Domain (TRBD), fingers, palm and thumb), organized into a "right hand" ring configuration that shares common features with
The protein consists of four conserved domains (RNA-Binding Domain (TRBD), fingers, palm and thumb), organized into a "right hand" ring configuration that shares common features with
 The lack of telomerase does not affect cell growth until the telomeres are short enough to cause cells to “die or undergo growth arrest”. However, inhibiting telomerase alone is not enough to destroy large tumors. It must be combined with surgery,
The lack of telomerase does not affect cell growth until the telomeres are short enough to cause cells to “die or undergo growth arrest”. However, inhibiting telomerase alone is not enough to destroy large tumors. It must be combined with surgery,
 Another independent approach is to use oligoadenylated anti-telomerase antisense
Another independent approach is to use oligoadenylated anti-telomerase antisense
Human telomerase reverse transcriptase (TERT) gene on genecards.org
The Telomerase Database - A Web-based tool for telomerase researchThree-dimensional model of telomerase
at MUN
Elizabeth Blackburn's Seminars: Telomeres and Telomerase
* * * {{Portal bar, Biology, border=no Ribonucleoproteins Aging-related enzymes Anti-aging substances DNA replication EC 2.7.7 Senescence Telomere-related proteins
 Telomerase, also called terminal transferase, is a
Telomerase, also called terminal transferase, is a ribonucleoprotein
Nucleoproteins are proteins conjugated with nucleic acids (either DNA or RNA). Typical nucleoproteins include ribosomes, nucleosomes and viral nucleocapsid proteins.
Structures
Nucleoproteins tend to be positively charged, facilitating int ...
that adds a species-dependent telomere repeat sequence to the 3' end of telomere
A telomere (; ) is a region of repetitive nucleotide sequences associated with specialized proteins at the ends of linear chromosomes. Although there are different architectures, telomeres, in a broad sense, are a widespread genetic feature mos ...
s. A telomere is a region of repetitive sequences
In mathematics, a sequence is an enumerated collection of objects in which repetitions are allowed and order matters. Like a set, it contains members (also called ''elements'', or ''terms''). The number of elements (possibly infinite) is called t ...
at each end of the chromosome
A chromosome is a long DNA molecule with part or all of the genetic material of an organism. In most chromosomes the very long thin DNA fibers are coated with packaging proteins; in eukaryotic cells the most important of these proteins are ...
s of most eukaryote
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bacte ...
s. Telomeres protect the end of the chromosome from DNA damage
DNA repair is a collection of processes by which a cell identifies and corrects damage to the DNA molecules that encode its genome. In human cells, both normal metabolic activities and environmental factors such as radiation can cause DNA da ...
or from fusion with neighbouring chromosomes. The fruit fly ''Drosophila melanogaster
''Drosophila melanogaster'' is a species of fly (the taxonomic order Diptera) in the family Drosophilidae. The species is often referred to as the fruit fly or lesser fruit fly, or less commonly the "vinegar fly" or "pomace fly". Starting with Ch ...
'' lacks telomerase, but instead uses retrotransposon
Retrotransposons (also called Class I transposable elements or transposons via RNA intermediates) are a type of genetic component that copy and paste themselves into different genomic locations (transposon) by converting RNA back into DNA through ...
s to maintain telomeres.
Telomerase is a reverse transcriptase
A reverse transcriptase (RT) is an enzyme used to generate complementary DNA (cDNA) from an RNA template, a process termed reverse transcription. Reverse transcriptases are used by viruses such as HIV and hepatitis B to replicate their genomes, ...
enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
that carries its own RNA molecule (e.g., with the sequence 3′- CCC AA UCCC-5′ in ''Trypanosoma brucei
''Trypanosoma brucei'' is a species of parasitic kinetoplastid belonging to the genus '' Trypanosoma'' that is present in sub-Saharan Africa. Unlike other protozoan parasites that normally infect blood and tissue cells, it is exclusively extrace ...
'') which is used as a template when it elongates telomeres. Telomerase is active in gamete
A gamete (; , ultimately ) is a haploid cell that fuses with another haploid cell during fertilization in organisms that reproduce sexually. Gametes are an organism's reproductive cells, also referred to as sex cells. In species that produce t ...
s and most cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
cells, but is normally absent from, or at very low levels in, most somatic cell
A somatic cell (from Ancient Greek σῶμα ''sôma'', meaning "body"), or vegetal cell, is any biological cell forming the body of a multicellular organism other than a gamete, germ cell, gametocyte or undifferentiated stem cell. Such cells compo ...
s.
History
The existence of a compensatory mechanism for telomere shortening was first found by Soviet biologistAlexey Olovnikov Alexey Matveyevich Olovnikov (russian: Алексей Матвеевич Оловников; 10 October 1936 in Vladivostok, Soviet Union – 6 December 2022 in Moscow, Russia) is a Russian biologist. In 1971, he was the first to recognize the probl ...
in 1973, who also suggested the telomere hypothesis of aging
Ageing ( BE) or aging ( AE) is the process of becoming older. The term refers mainly to humans, many other animals, and fungi, whereas for example, bacteria, perennial plants and some simple animals are potentially biologically immortal. In ...
and the telomere's connections to cancer.
Telomerase in the ciliate ''Tetrahymena
''Tetrahymena'', a Unicellular organism, unicellular eukaryote, is a genus of free-living ciliates. The genus Tetrahymena is the most widely studied member of its phylum. It can produce, store and react with different types of hormones. Tetrah ...
'' was discovered by Carol W. Greider
Carolyn Widney Greider (born April 15, 1961) is an American molecular biologist and Nobel laureate. She joined the University of California, Santa Cruz as a Distinguished Professor in the department of molecular, cell, and developmental biology ...
and Elizabeth Blackburn
Elizabeth Helen Blackburn, (born 26 November 1948) is an Australian-American Nobel laureate who is the former president of the Salk Institute for Biological Studies. Previously she was a biological researcher at the University of California, ...
in 1984. Together with Jack W. Szostak
Jack William Szostak (born November 9, 1952) is a Canadian American biologist of Polish British descent, Nobel Prize laureate, university professor at the University of Chicago, former Professor of Genetics at Harvard Medical School, and Alexan ...
, Greider and Blackburn were awarded the 2009 Nobel Prize
The Nobel Prizes ( ; sv, Nobelpriset ; no, Nobelprisen ) are five separate prizes that, according to Alfred Nobel's will of 1895, are awarded to "those who, during the preceding year, have conferred the greatest benefit to humankind." Alfr ...
in Physiology or Medicine
The Nobel Prize in Physiology or Medicine is awarded yearly by the Nobel Assembly at the Karolinska Institute for outstanding discoveries in physiology or medicine. The Nobel Prize is not a single prize, but five separate prizes that, according ...
for their discovery.
The role of telomeres and telomerase in cell aging and cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
was established by scientists at biotechnology
Biotechnology is the integration of natural sciences and engineering sciences in order to achieve the application of organisms, cells, parts thereof and molecular analogues for products and services. The term ''biotechnology'' was first used b ...
company Geron with the cloning of the RNA
Ribonucleic acid (RNA) is a polymeric molecule essential in various biological roles in coding, decoding, regulation and expression of genes. RNA and deoxyribonucleic acid ( DNA) are nucleic acids. Along with lipids, proteins, and carbohydra ...
and catalytic components of human telomerase and the development of a polymerase chain reaction
The polymerase chain reaction (PCR) is a method widely used to rapidly make millions to billions of copies (complete or partial) of a specific DNA sample, allowing scientists to take a very small sample of DNA and amplify it (or a part of it) t ...
(PCR) based assay for telomerase activity called the TRAP assay, which surveys telomerase activity in multiple types of cancer.
The negative stain In microscopy, negative staining is an established method, often used in diagnostic microscopy, for contrasting a thin specimen with an optically opaque fluid. In this technique, the background is stained, leaving the actual specimen untouched, and ...
electron microscopy (EM) structures of human and ''Tetrahymena'' telomerases were characterized in 2013. Two years later, the first cryo-electron microscopy (cryo-EM
Cryogenic electron microscopy (cryo-EM) is a cryomicroscopy technique applied on samples cooled to cryogenic temperatures. For biological specimens, the structure is preserved by embedding in an environment of vitreous ice. An aqueous sample so ...
) structure of telomerase holoenzyme (''Tetrahymena'') was determined. In 2018, the structure of human telomerase was determined through cryo-EM by UC Berkeley scientists.
Human telomerase structure
The molecular composition of the human telomerase complex was determined by Scott Cohen and his team at the Children's Medical Research Institute (Sydney Australia) and consists of two molecules each of humantelomerase reverse transcriptase
Telomerase reverse transcriptase (abbreviated to TERT, or hTERT in humans) is a catalytic subunit of the enzyme telomerase, which, together with the telomerase RNA component (TERC), comprises the most important unit of the telomerase complex.
T ...
(TERT), telomerase RNA (TR or TERC), and dyskerin
H/ACA ribonucleoprotein complex subunit 4 is a protein that in humans is encoded by the gene ''DKC1''.
This gene is a member of the snoRNA, H/ACA snoRNPs (small nucleolar ribonucleoproteins) gene family. snoRNPs are involved in various aspects of ...
(DKC1). The genes of telomerase subunits, which include TERT, TERC, DKC1 and TEP1, are located on different chromosomes. The human TERT gene (hTERT) is translated
Translation is the communication of the meaning of a source-language text by means of an equivalent target-language text. The English language draws a terminological distinction (which does not exist in every language) between ''transla ...
into a protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, respo ...
of 1132 amino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
. TERT polypeptide folds with (and carries) TERC, a non-coding RNA
A non-coding RNA (ncRNA) is a functional RNA molecule that is not Translation (genetics), translated into a protein. The DNA sequence from which a functional non-coding RNA is transcribed is often called an RNA gene. Abundant and functionally im ...
(451 nucleotide
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules wi ...
s long). TERT has a 'mitten' structure that allows it to wrap around the chromosome to add single-stranded telomere repeats.
TERT is a reverse transcriptase
A reverse transcriptase (RT) is an enzyme used to generate complementary DNA (cDNA) from an RNA template, a process termed reverse transcription. Reverse transcriptases are used by viruses such as HIV and hepatitis B to replicate their genomes, ...
, which is a class of enzymes that creates single-stranded DNA using single-stranded RNA as a template.
 The protein consists of four conserved domains (RNA-Binding Domain (TRBD), fingers, palm and thumb), organized into a "right hand" ring configuration that shares common features with
The protein consists of four conserved domains (RNA-Binding Domain (TRBD), fingers, palm and thumb), organized into a "right hand" ring configuration that shares common features with retroviral
A retrovirus is a type of virus that inserts a DNA copy of its RNA genome into the DNA of a host cell that it invades, thus changing the genome of that cell. Once inside the host cell's cytoplasm, the virus uses its own reverse transcriptas ...
reverse transcriptases, viral RNA replicase
RNA-dependent RNA polymerase (RdRp) or RNA replicase is an enzyme that catalyzes the replication of RNA from an RNA template. Specifically, it catalyzes synthesis of the RNA strand complementary to a given RNA template. This is in contrast to ...
s and bacteriophage
A bacteriophage (), also known informally as a ''phage'' (), is a duplodnaviria virus that infects and replicates within bacteria and archaea. The term was derived from "bacteria" and the Greek φαγεῖν ('), meaning "to devour". Bacteri ...
B-family DNA polymerases.
TERT proteins from many eukaryotes have been sequenced.
Mechanism
By using TERC, TERT can add a six-nucleotide repeating sequence, 5'- TTA GGG (in vertebrates, the sequence differs in other organisms) to the 3' strand of chromosomes. These TTAGGG repeats (with their various protein binding partners) are called telomeres. The template region of TERC is 3'-CAAUCCCAAUC-5'. Telomerase can bind the first few nucleotides of the template to the last telomere sequence on the chromosome, add a new telomere repeat (5'-GGTTAG-3') sequence, let go, realign the new 3'-end of telomere to the template, and repeat the process. Telomerase reversestelomere shortening
A telomere (; ) is a region of repetitive nucleotide sequences associated with specialized proteins at the ends of linear chromosomes. Although there are different architectures, telomeres, in a broad sense, are a widespread genetic feature mos ...
.
Clinical implications
Aging
Telomerase restores short bits of DNA known astelomeres
A telomere (; ) is a region of repetitive nucleotide sequences associated with specialized proteins at the ends of linear chromosomes. Although there are different architectures, telomeres, in a broad sense, are a widespread genetic feature mos ...
, which are otherwise shortened after repeated division of a cell via mitosis
In cell biology, mitosis () is a part of the cell cycle in which replicated chromosomes are separated into two new nuclei. Cell division by mitosis gives rise to genetically identical cells in which the total number of chromosomes is mainta ...
.
In normal circumstances, where telomerase is absent, if a cell divides recursively, at some point the progeny reach their Hayflick limit, which is believed to be between 50 and 70 cell divisions. At the limit the cells become senescent and cell division
Cell division is the process by which a parent cell (biology), cell divides into two daughter cells. Cell division usually occurs as part of a larger cell cycle in which the cell grows and replicates its chromosome(s) before dividing. In eukar ...
stops. Telomerase allows each offspring to replace the lost bit of DNA, allowing the cell line to divide without ever reaching the limit. This same unbounded growth is a feature of cancerous growth.
Embryonic stem cells
Embryonic stem cells (ESCs) are pluripotent stem cells derived from the inner cell mass of a blastocyst, an early-stage pre- implantation embryo. Human embryos reach the blastocyst stage 4–5 days post fertilization, at which time they consist ...
express telomerase, which allows them to divide repeatedly and form the individual. In adults, telomerase is highly expressed only in cells that need to divide regularly, especially in male sperm cell
Sperm is the male reproductive cell, or gamete, in anisogamous forms of sexual reproduction (forms in which there is a larger, female reproductive cell and a smaller, male one). Animals produce motile sperm with a tail known as a flagellum, whi ...
s, but also in epidermal cells
The epidermis is the outermost of the three layers that comprise the skin, the inner layers being the dermis and hypodermis. The epidermis layer provides a barrier to infection from environmental pathogens and regulates the amount of water relea ...
, in activated T cell
A T cell is a type of lymphocyte. T cells are one of the important white blood cells of the immune system and play a central role in the adaptive immune response. T cells can be distinguished from other lymphocytes by the presence of a T-cell r ...
and B cell lymphocyte
A lymphocyte is a type of white blood cell (leukocyte) in the immune system of most vertebrates. Lymphocytes include natural killer cells (which function in cell-mediated, cytotoxic innate immunity), T cells (for cell-mediated, cytotoxic ad ...
s, as well as in certain adult stem cells
Adult stem cells are undifferentiated cells, found throughout the body after development, that multiply by cell division to replenish dying cells and regenerate damaged tissues. Also known as somatic stem cells (from Greek σωματικóς, ...
, but in the great majority of cases somatic cells do not express telomerase.
A comparative biology study of mammalian telomeres indicated that telomere length of some mammalian species correlates inversely, rather than directly, with lifespan, and concluded that the contribution of telomere length to lifespan is unresolved. Telomere shortening does not occur with age in some postmitotic
The G0 phase describes a cellular state outside of the replicative cell cycle. Classically, cells were thought to enter G0 primarily due to environmental factors, like nutrient deprivation, that limited the resources necessary for proliferation ...
tissues, such as in the rat brain. In humans, skeletal muscle telomere lengths remain stable from ages 23 –74. In baboon skeletal muscle, which consists of fully differentiated postmitotic cells, less than 3% of myonuclei
Skeletal muscles (commonly referred to as muscles) are organs of the vertebrate muscular system and typically are attached by tendons to bones of a skeleton. The muscle cells of skeletal muscles are much longer than in the other types of muscle ...
contain damaged telomeres and this percentage does not increase with age. Thus, telomere shortening does not appear to be a major factor in the aging of the differentiated cells of brain or skeletal muscle. In human liver, cholangiocytes
Cholangiocytes are the epithelial cells of the bile duct. They are cuboidal epithelium in the small interlobular bile ducts, but become columnar and HCO3:-secreting in larger bile ducts approaching the porta hepatis and the extrahepatic ducts. T ...
and hepatocytes
A hepatocyte is a cell of the main parenchymal tissue of the liver. Hepatocytes make up 80% of the liver's mass.
These cells are involved in:
* Protein synthesis
* Protein storage
* Transformation of carbohydrates
* Synthesis of cholesterol, ...
show no age-related telomere shortening. Another study found little evidence that, in humans, telomere length is a significant biomarker
In biomedical contexts, a biomarker, or biological marker, is a measurable indicator of some biological state or condition. Biomarkers are often measured and evaluated using blood, urine, or soft tissues to examine normal biological processes, p ...
of normal aging with respect to important cognitive and physical abilities.
Some experiments have raised questions on whether telomerase can be used as an anti-aging therapy, namely, the fact that mice with elevated levels of telomerase have higher cancer incidence and hence do not live longer. On the other hand, one study showed that activating telomerase in cancer-resistant mice by overexpressing its catalytic subunit extended lifespan. A study found that long-lived subjects inherited a hyperactive version of telomerase.
*
Premature aging
Premature aging syndromes includingWerner syndrome
Werner syndrome (WS) or Werner's syndrome, also known as "adult progeria",James, William; Berger, Timothy; Elston, Dirk (2005). ''Andrews' Diseases of the Skin: Clinical Dermatology''. (10th ed.). Saunders. . is a rare, autosomal recessive disorder ...
, Progeria
Progeria is a specific type of progeroid syndrome, also known as Hutchinson–Gilford syndrome. A single gene mutation is responsible for progeria. The gene, known as lamin A (LMNA), makes a protein necessary for holding the Nucleus of the cell ...
, Ataxia telangiectasia
Ataxia is a neurological sign consisting of lack of voluntary Motor coordination, coordination of muscle movements that can include gait abnormality, speech changes, and abnormalities in eye movements. Ataxia is a clinical manifestation indicati ...
, Ataxia-telangiectasia like disorder, Bloom syndrome
Bloom syndrome (often abbreviated as BS in literature) is a rare autosomal recessive genetic disorder characterized by short stature, predisposition to the development of cancer, and genomic instability. BS is caused by mutations in the '' BLM'' ge ...
, Fanconi anemia
Fanconi anaemia (FA) is a rare genetic disease resulting in impaired response to DNA damage. Although it is a very rare disorder, study of this and other bone marrow failure syndromes has improved scientific understanding of the mechanisms of no ...
and Nijmegen breakage syndrome are associated with short telomeres. However, the genes that have mutated in these diseases all have roles in the repair of DNA damage and the increased DNA damage may, itself, be a factor in the premature aging (see DNA damage theory of aging
The DNA damage theory of aging proposes that aging is a consequence of unrepaired accumulation of naturally occurring DNA damage. Damage in this context is a DNA alteration that has an abnormal structure. Although both mitochondrial and nuclear ...
). An additional role in maintaining telomere length is an active area of investigation.
Cancer
''In vitro,'' when cells approach the Hayflick limit, the time to senescence can be extended by inactivating thetumor suppressor
A tumor suppressor gene (TSG), or anti-oncogene, is a gene that regulates a cell during cell division and replication. If the cell grows uncontrollably, it will result in cancer. When a tumor suppressor gene is mutated, it results in a loss or re ...
proteins - p53
p53, also known as Tumor protein P53, cellular tumor antigen p53 (UniProt name), or transformation-related protein 53 (TRP53) is a regulatory protein that is often mutated in human cancers. The p53 proteins (originally thought to be, and often s ...
and Retinoblastoma protein
The retinoblastoma protein (protein name abbreviated pRb; gene name abbreviated ''Rb'', ''RB'' or ''RB1'') is a proto-oncogenic tumor suppressor protein that is dysfunctional in several major cancers. One function of pRb is to prevent excessive ...
(pRb). Cell
Cell most often refers to:
* Cell (biology), the functional basic unit of life
Cell may also refer to:
Locations
* Monastic cell, a small room, hut, or cave in which a religious recluse lives, alternatively the small precursor of a monastery ...
s that have been so-altered eventually undergo an event termed a "crisis" when the majority of the cells in the culture die. Sometimes, a cell does not stop dividing once it reaches a crisis. In a typical situation, the telomeres are shortened and chromosomal integrity declines with every subsequent cell division. Exposed chromosome ends are interpreted as double-stranded breaks (DSB) in DNA; such damage is usually repaired by reattaching the broken ends together. When the cell does this due to telomere-shortening, the ends of different chromosomes can be attached to each other. This solves the problem of lacking telomeres, but during cell division anaphase
Anaphase () is the stage of mitosis after the process of metaphase, when replicated chromosomes are split and the newly-copied chromosomes (daughter chromatids) are moved to opposite poles of the cell. Chromosomes also reach their overall maxim ...
, the fused chromosomes are randomly ripped apart, causing many mutation
In biology, a mutation is an alteration in the nucleic acid sequence of the genome of an organism, virus, or extrachromosomal DNA. Viral genomes contain either DNA or RNA. Mutations result from errors during DNA or viral replication, mi ...
s and chromosomal abnormalities. As this process continues, the cell's genome becomes unstable. Eventually, either fatal damage is done to the cell's chromosomes (killing it via apoptosis
Apoptosis (from grc, ἀπόπτωσις, apóptōsis, 'falling off') is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes (morphology) and death. These changes incl ...
), or an additional mutation that activates telomerase occurs.
With telomerase activation some types of cells and their offspring become immortal
Immortality is the ability to live forever, or eternal life.
Immortal or Immortality may also refer to:
Film
* ''The Immortals'' (1995 film), an American crime film
* ''Immortality'', an alternate title for the 1998 British film ''The Wisdom of ...
(bypass the Hayflick limit), thus avoiding cell death as long as the conditions for their duplication are met. Many cancer cell
Cancer cells are cells that divide continually, forming solid tumors or flooding the blood with abnormal cells. Cell division is a normal process used by the body for growth and repair. A parent cell divides to form two daughter cells, and these d ...
s are considered 'immortal' because telomerase activity allows them to live much longer than any other somatic cell, which, combined with uncontrollable cell proliferation is why they can form tumor
A neoplasm () is a type of abnormal and excessive growth of tissue. The process that occurs to form or produce a neoplasm is called neoplasia. The growth of a neoplasm is uncoordinated with that of the normal surrounding tissue, and persists ...
s. A good example of immortal cancer cells is HeLa cell
HeLa (; also Hela or hela) is an immortalized cell line used in scientific research. It is the oldest and most commonly used human cell line. The line is derived from cervical cancer cells taken on February 8, 1951, named after Henrietta L ...
s, which have been used in laboratories as a model cell line
An immortalised cell line is a population of cells from a multicellular organism which would normally not proliferate indefinitely but, due to mutation, have evaded normal cellular senescence and instead can keep undergoing division. The cell ...
since 1951.
While this method of modelling human cancer in cell culture is effective and has been used for many years by scientists, it is also very imprecise. The exact changes that allow for the formation of the tumorigenic
Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cells are transformed into cancer cells. The process is characterized by changes at the cellular, genetic, and epigenetic levels and abno ...
clones in the above-described experiment are not clear. Scientists addressed this question by the serial introduction of multiple mutations present in a variety of human cancers. This has led to the identification of mutation combinations that form tumorigenic cells in a variety of cell types. While the combination varies by cell type, the following alterations are required in all cases: TERT activation, loss of p53 pathway function, loss of pRb pathway function, activation of the Ras
Ras or RAS may refer to:
Arts and media
* RAS Records Real Authentic Sound, a reggae record label
* Rundfunk Anstalt Südtirol, a south Tyrolese public broadcasting service
* Rás 1, an Icelandic radio station
* Rás 2, an Icelandic radio stati ...
or myc
''Myc'' is a family of regulator genes and proto-oncogenes that code for transcription factors. The ''Myc'' family consists of three related human genes: ''c-myc'' (MYC), ''l-myc'' ( MYCL), and ''n-myc'' (MYCN). ''c-myc'' (also sometimes refe ...
proto-oncogenes
An oncogene is a gene that has the potential to cause cancer. In tumor cells, these genes are often mutated, or expressed at high levels.
, and aberration of the PP2A protein phosphatase
In biochemistry, a phosphatase is an enzyme that uses water to cleave a phosphoric acid Ester, monoester into a phosphate ion and an Alcohol (chemistry), alcohol. Because a phosphatase enzyme catalysis, catalyzes the hydrolysis of its Substrate ...
. That is to say, the cell has an activated telomerase, eliminating the process of death by chromosome instability or loss, absence of apoptosis-induction pathways, and continued mitosis
In cell biology, mitosis () is a part of the cell cycle in which replicated chromosomes are separated into two new nuclei. Cell division by mitosis gives rise to genetically identical cells in which the total number of chromosomes is mainta ...
activation.
This model of cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
in cell culture accurately describes the role of telomerase in actual human tumors. Telomerase activation has been observed in ~90% of all human tumors, suggesting that the immortality conferred by telomerase plays a key role in cancer development. Of the tumors without TERT activation, most employ a separate pathway to maintain telomere length termed Alternative Lengthening of Telomeres (ALT). The exact mechanism behind telomere maintenance in the ALT pathway is unclear, but likely involves multiple recombination events at the telomere.
Elizabeth Blackburn
Elizabeth Helen Blackburn, (born 26 November 1948) is an Australian-American Nobel laureate who is the former president of the Salk Institute for Biological Studies. Previously she was a biological researcher at the University of California, ...
''et al.'', identified the upregulation of 70 genes known or suspected in cancer growth and spread through the body, and the activation of glycolysis, which enables cancer cells to rapidly use sugar to facilitate their programmed growth rate (roughly the growth rate of a fetus).
Approaches to controlling telomerase and telomeres for cancer therapy include gene therapy
Gene therapy is a medical field which focuses on the genetic modification of cells to produce a therapeutic effect or the treatment of disease by repairing or reconstructing defective genetic material. The first attempt at modifying human DN ...
, immunotherapy
Immunotherapy or biological therapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as ''activation immunotherapies,'' while immunotherap ...
, small-molecule and signal pathway inhibitors.
Drugs
The ability to maintain functionaltelomeres
A telomere (; ) is a region of repetitive nucleotide sequences associated with specialized proteins at the ends of linear chromosomes. Although there are different architectures, telomeres, in a broad sense, are a widespread genetic feature mos ...
may be one mechanism that allows cancer cells to grow ''in vitro'' for decades. Telomerase activity is necessary to preserve many cancer types and is inactive in somatic cells
A somatic cell (from Ancient Greek σῶμα ''sôma'', meaning "body"), or vegetal cell, is any biological cell forming the body of a multicellular organism other than a gamete, germ cell, gametocyte or undifferentiated stem cell. Such cells compo ...
, creating the possibility that telomerase inhibition could selectively repress cancer cell growth with minimal side effects. If a drug can inhibit telomerase in cancer cells, the telomeres of successive generations will progressively shorten, limiting tumor growth.
Telomerase is a good biomarker
In biomedical contexts, a biomarker, or biological marker, is a measurable indicator of some biological state or condition. Biomarkers are often measured and evaluated using blood, urine, or soft tissues to examine normal biological processes, p ...
for cancer detection because most human cancers cells express high levels of it. Telomerase activity can be identified by its catalytic protein domain ( hTERT). is the rate-limiting step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of th ...
in telomerase activity. It is associated with many cancer types. Various cancer cells and fibroblasts
A fibroblast is a type of biological cell that synthesizes the extracellular matrix and collagen, produces the structural framework ( stroma) for animal tissues, and plays a critical role in wound healing. Fibroblasts are the most common cells o ...
transformed with hTERT cDNA have high telomerase activity, while somatic cells do not. Cells testing positive for hTERT have positive nuclear signals. Epithelial stem cell tissue and its early daughter cells are the only noncancerous cells in which hTERT can be detected. Since hTERT expression is dependent only on the number of tumor cells within a sample, the amount of hTERT indicates the severity of cancer.
The expression of hTERT can also be used to distinguish benign tumors
A benign tumor is a mass of cells (tumor) that does not invade neighboring tissue or metastasize (spread throughout the body). Compared to malignant (cancerous) tumors, benign tumors generally have a slower growth rate. Benign tumors have rel ...
from malignant tumors
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal bl ...
. Malignant tumors have higher hTERT expression than benign tumors. Real-time reverse transcription polymerase chain reaction (RT-PCR) quantifying hTERT expression in various tumor samples verified this varying expression.
 The lack of telomerase does not affect cell growth until the telomeres are short enough to cause cells to “die or undergo growth arrest”. However, inhibiting telomerase alone is not enough to destroy large tumors. It must be combined with surgery,
The lack of telomerase does not affect cell growth until the telomeres are short enough to cause cells to “die or undergo growth arrest”. However, inhibiting telomerase alone is not enough to destroy large tumors. It must be combined with surgery, radiation
In physics, radiation is the emission or transmission of energy in the form of waves or particles through space or through a material medium. This includes:
* ''electromagnetic radiation'', such as radio waves, microwaves, infrared, visi ...
, chemotherapy
Chemotherapy (often abbreviated to chemo and sometimes CTX or CTx) is a type of cancer treatment that uses one or more anti-cancer drugs (chemotherapeutic agents or alkylating agents) as part of a standardized chemotherapy regimen. Chemotherap ...
or immunotherapy.
Cells may reduce their telomere length by only 50-252 base pairs per cell division, which can lead to a long lag phase
250px, Growth is shown as ''L'' = log(numbers) where numbers is the number of colony forming units per ml, versus ''T'' (time.)
Bacterial growth is proliferation of bacterium into two daughter cells, in a process called binary fission. Providing ...
.
A telomerase activator TA-65 is commercially available and is claimed to delay aging and to provide relief from certain disease conditions. This formulation contains a molecule called cycloastragenol
Cycloastragenol is a triterpenoid isolated from various legume species in the genus ''Astragalus'' that is purported to have telomerase activation activity. A preliminary ''in vitro'' study on human CD4 and CD8 T cells found that cycloastragenol ...
derived from a legume Astragalus membranaceus, which has been used in Chinese traditional medicine for extending healthy life.
Immunotherapy
Immunotherapy
Immunotherapy or biological therapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as ''activation immunotherapies,'' while immunotherap ...
successfully treats some kinds of cancer, such as melanoma
Melanoma, also redundantly known as malignant melanoma, is a type of skin cancer that develops from the pigment-producing cells known as melanocytes. Melanomas typically occur in the skin, but may rarely occur in the mouth, intestines, or eye ( ...
. This treatment involves manipulating a human's immune system
The immune system is a network of biological processes that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to parasitic worms, as well as cancer cells and objects such as wood splinte ...
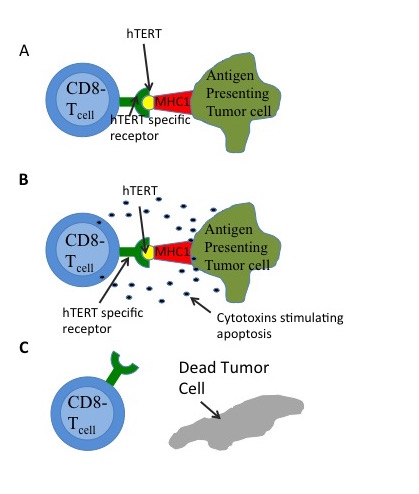
to destroy cancerous cells. Humans have two major antigen
In immunology, an antigen (Ag) is a molecule or molecular structure or any foreign particulate matter or a pollen grain that can bind to a specific antibody or T-cell receptor. The presence of antigens in the body may trigger an immune response. ...
identifying lymphocyte
A lymphocyte is a type of white blood cell (leukocyte) in the immune system of most vertebrates. Lymphocytes include natural killer cells (which function in cell-mediated, cytotoxic innate immunity), T cells (for cell-mediated, cytotoxic ad ...
s: CD8
CD8 (cluster of differentiation 8) is a transmembrane glycoprotein that serves as a co-receptor for the T-cell receptor (TCR). Along with the TCR, the CD8 co-receptor plays a role in T cell signaling and aiding with cytotoxic T cell-antigen int ...
+ cytotoxic T-lymphocytes
A cytotoxic T cell (also known as TC, cytotoxic T lymphocyte, CTL, T-killer cell, cytolytic T cell, CD8+ T-cell or killer T cell) is a T lymphocyte (a type of white blood cell) that kills cancer cells, cells that are infected by intracellular p ...
(CTL) and CD4+ helper T-lymphocytes that can destroy cells. Antigen receptors on CTL can bind to a 9-10 amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
chain that is presented by the major histocompatibility complex
The major histocompatibility complex (MHC) is a large locus on vertebrate DNA containing a set of closely linked polymorphic genes that code for cell surface proteins essential for the adaptive immune system. These cell surface proteins are calle ...
(MHC) as in Figure 4. HTERT is a potential target antigen. Immunotargeting should result in relatively few side effects since hTERT expression is associated only with telomerase and is not essential in almost all somatic cells. GV1001 uses this pathway. Experimental drug and vaccine
A vaccine is a biological Dosage form, preparation that provides active acquired immunity to a particular infectious disease, infectious or cancer, malignant disease. The safety and effectiveness of vaccines has been widely studied and verifie ...
therapies targeting active telomerase have been tested in mouse model
A model organism (often shortened to model) is a non-human species that is extensively studied to understand particular biological phenomena, with the expectation that discoveries made in the model organism will provide insight into the working ...
s, and clinical trial
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietar ...
s have begun. One drug, imetelstat
Imetelstat (development code GRN163L) is an experimental anticancer drug. The first telomerase inhibitor to enter clinical trials, As of early 2023, it was in Phase 2/ 3 trials for various cancer types.
Chemically, imetelstat is a synthetic co ...
, is being clinically researched as a means of interfering with telomerase in cancer cells. Most of the harmful cancer-related effects of telomerase are dependent on an intact RNA template. Cancer stem cells that use an alternative method of telomere maintenance are still killed when telomerase's RNA template is blocked or damaged.
Telomerase Vaccines
Two telomerase vaccines have been developed: GRNVAC1 and GV1001. GRNVAC1 isolatesdendritic cells
Dendritic cells (DCs) are antigen-presenting cells (also known as ''accessory cells'') of the mammalian immune system. Their main function is to process antigen material and present it on the cell surface to the T cells of the immune system. The ...
and the RNA that codes for the telomerase protein and puts them back into the patient to make cytotoxic
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are an immune cell or some types of venom, e.g. from the puff adder (''Bitis arietans'') or brown recluse spider (''Loxosceles reclusa'').
Cell physiology
Treating cells ...
T cells that kill the telomerase-active cells. GV1001 is a peptide from the active site of hTERT and is recognized by the immune system that reacts by killing the telomerase-active cells.
Targeted apoptosis
 Another independent approach is to use oligoadenylated anti-telomerase antisense
Another independent approach is to use oligoadenylated anti-telomerase antisense oligonucleotides
Oligonucleotides are short DNA or RNA molecules, oligomers, that have a wide range of applications in genetic testing, research, and forensics. Commonly made in the laboratory by solid-phase chemical synthesis, these small bits of nucleic acids ...
and ribozymes
Ribozymes (ribonucleic acid enzymes) are RNA molecules that have the ability to catalyze specific biochemical reactions, including RNA splicing in gene expression, similar to the action of protein enzymes. The 1982 discovery of ribozymes demonst ...
to target telomerase RNA, reducing dissociation and apoptosis
Apoptosis (from grc, ἀπόπτωσις, apóptōsis, 'falling off') is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes (morphology) and death. These changes incl ...
(Figure 5). The fast induction of apoptosis through antisense binding may be a good alternative to the slower telomere shortening.
Small interfering RNA (siRNA)
siRNAs are small RNA molecules that induce the sequence-specific degradation of other RNAs. siRNA treatment can function similar to traditionalgene therapy
Gene therapy is a medical field which focuses on the genetic modification of cells to produce a therapeutic effect or the treatment of disease by repairing or reconstructing defective genetic material. The first attempt at modifying human DN ...
by destroying the mRNA
In molecular biology, messenger ribonucleic acid (mRNA) is a single-stranded molecule of RNA that corresponds to the genetic sequence of a gene, and is read by a ribosome in the process of Protein biosynthesis, synthesizing a protein.
mRNA is ...
products of particular genes, and therefore preventing the expression of those genes. A 2012 study found that targeting TERC with an siRNA reduced telomerase activity by more than 50% and resulted in decreased viability of immortal cancer cells. Treatment with both the siRNA and radiation caused a greater reduction in tumor size in mice than treatment with radiation alone, suggesting that targeting telomerase could be a way to increase the efficacy of radiation in treating radiation-resistant tumors.
Heart disease, diabetes and quality of life
Blackburn also discovered that mothers caring for very sick children have shorter telomeres when they report that their emotional stress is at a maximum and that telomerase was active at the site of blockages incoronary artery
The coronary arteries are the arterial blood vessels of coronary circulation, which transport oxygenated blood to the heart muscle. The heart requires a continuous supply of oxygen to function and survive, much like any other tissue or organ ...
tissue, possibly accelerating heart attacks.
In 2009, it was shown that the amount of telomerase activity significantly increased following psychological stress
In psychology, stress is a feeling of emotional strain and pressure. Stress is a type of psychological pain. Small amounts of stress may be beneficial, as it can improve athletic performance, motivation and reaction to the environment. Exces ...
. Across the sample of patients telomerase activity in peripheral blood mononuclear cell
A peripheral blood mononuclear cell (PBMC) is any peripheral blood cell having a round nucleus. These cells consist of lymphocytes (T cells, B cells, NK cells) and monocytes, whereas erythrocytes and platelets have no nuclei, and granulocytes ( ...
s increased by 18% one hour after the end of the stress.
A study in 2010 found that there was "significantly greater" telomerase activity in participants than controls after a three-month meditation retreat.
Telomerase deficiency has been linked to diabetes
Diabetes, also known as diabetes mellitus, is a group of metabolic disorders characterized by a high blood sugar level ( hyperglycemia) over a prolonged period of time. Symptoms often include frequent urination, increased thirst and increased ap ...
mellitus and impaired insulin
Insulin (, from Latin ''insula'', 'island') is a peptide hormone produced by beta cells of the pancreatic islets encoded in humans by the ''INS'' gene. It is considered to be the main anabolic hormone of the body. It regulates the metabolism o ...
secretion in mice, due to loss of pancreatic insulin-producing cells.
Rare human diseases
Mutations in TERT have been implicated in predisposing patients toaplastic anemia
Aplastic anemia is a cancer in which the body fails to make blood cells in sufficient numbers. Blood cells are produced in the bone marrow by stem cells that reside there. Aplastic anemia causes a deficiency of all blood cell types: red blood ...
, a disorder in which the bone marrow
Bone marrow is a semi-solid tissue found within the spongy (also known as cancellous) portions of bones. In birds and mammals, bone marrow is the primary site of new blood cell production (or haematopoiesis). It is composed of hematopoietic ce ...
fails to produce blood cells, in 2005.
Cri du chat syndrome
Cri du chat syndrome is a rare genetic disorder due to a partial chromosome deletion on chromosome 5. Its name is a French term ("cat-cry" or " call of the cat") referring to the characteristic cat-like cry of affected children. It was first de ...
(CdCS) is a complex disorder involving the loss of the distal portion of the short arm of chromosome 5
Chromosome 5 is one of the 23 pairs of chromosomes in humans. People normally have two copies of this chromosome. Chromosome 5 spans about 181 million base pairs (the building blocks of DNA) and represents almost 6% of the total DNA in cells. C ...
. TERT is located in the deleted region, and loss of one copy of TERT has been suggested as a cause or contributing factor of this disease.
Dyskeratosis congenita
Dyskeratosis congenita (DKC), also known as Zinsser-Engman-Cole syndrome, is a rare progressive congenital disorder with a highly variable phenotype. The entity was classically defined by the triad of abnormal skin pigmentation, nail dystrophy, and ...
(DC) is a disease of the bone marrow
Bone marrow is a semi-solid tissue found within the spongy (also known as cancellous) portions of bones. In birds and mammals, bone marrow is the primary site of new blood cell production (or haematopoiesis). It is composed of hematopoietic ce ...
that can be caused by some mutations in the telomerase subunits. In the DC cases, about 35% cases are X-linked
Sex linked describes the sex-specific patterns of inheritance and presentation when a gene mutation (allele) is present on a sex chromosome (allosome) rather than a non-sex chromosome (autosome). In humans, these are termed X-linked recessive, ...
-recessive
In genetics, dominance is the phenomenon of one variant (allele) of a gene on a chromosome masking or overriding the effect of a different variant of the same gene on the other copy of the chromosome. The first variant is termed dominant and t ...
on the DKC1 locus and 5% cases are autosomal
An autosome is any chromosome that is not a sex chromosome. The members of an autosome pair in a diploid cell have the same morphology, unlike those in allosomal (sex chromosome) pairs, which may have different structures. The DNA in autosom ...
dominant on the TERT and TERC loci.
Patients with DC have severe bone marrow failure manifesting as abnormal skin pigmentation
Human skin color ranges from the darkest brown to the lightest hues. Differences in skin color among individuals is caused by variation in pigmentation, which is the result of genetics (inherited from one's biological parents and or individu ...
, leucoplakia
Oral leukoplakia is a ''potentially malignant disorder'' affecting the oral mucosa. It is defined as "essentially an oral mucosal white lesion that cannot be considered as any other definable lesion." Oral leukoplakia is a white patch or plaque th ...
(a white thickening of the oral mucosa) and nail dystrophy
A nail disease or onychosis is a disease or deformity of the nail. Although the nail is a structure produced by the skin and is a skin appendage, nail diseases have a distinct classification as they have their own signs and symptoms which may r ...
, as well as a variety of other symptoms. Individuals with either TERC or DKC1 mutations have shorter telomeres and defective telomerase activity ''in vitro'' versus other individuals of the same age.
In one family autosomal dominant DC was linked to a heterozygous
Zygosity (the noun, zygote, is from the Greek "yoked," from "yoke") () is the degree to which both copies of a chromosome or gene have the same genetic sequence. In other words, it is the degree of similarity of the alleles in an organism.
Mo ...
TERT mutation. These patients also exhibited an increased rate of telomere-shortening, and genetic anticipation In genetics, anticipation is a phenomenon whereby as a genetic disorder is passed on to the next generation, the symptoms of the genetic disorder become apparent at an earlier age with each generation. In most cases, an increase in the severity of ...
(i.e., the DC phenotype worsened with each generation).
TERT Splice Variants
See also
*DNA repair
DNA repair is a collection of processes by which a cell identifies and corrects damage to the DNA molecules that encode its genome. In human cells, both normal metabolic activities and environmental factors such as radiation can cause DNA dam ...
*Imetelstat
Imetelstat (development code GRN163L) is an experimental anticancer drug. The first telomerase inhibitor to enter clinical trials, As of early 2023, it was in Phase 2/ 3 trials for various cancer types.
Chemically, imetelstat is a synthetic co ...
* TA-65
*Telomere
A telomere (; ) is a region of repetitive nucleotide sequences associated with specialized proteins at the ends of linear chromosomes. Although there are different architectures, telomeres, in a broad sense, are a widespread genetic feature mos ...
* Epitalon
Reference
Further reading
* ''The Immortal Cell'', byMichael D. West
Michael D. West (born in Niles, Michigan on 28 April 1953) is an American biogerontologist, and a pioneer in stem cells, cellular aging and telomerase. He is the founder and CEO of AgeX Therapeutics,
a startup focused on the field of experime ...
, Doubleday (2003)
External links
*Gene Ontology
The Gene Ontology (GO) is a major bioinformatics initiative to unify the representation of gene and gene product attributes across all species. More specifically, the project aims to: 1) maintain and develop its controlled vocabulary of gene and g ...
: Human telomerase reverse transcriptase (TERT) gene on genecards.org
The Telomerase Database - A Web-based tool for telomerase research
at MUN
Elizabeth Blackburn's Seminars: Telomeres and Telomerase
* * * {{Portal bar, Biology, border=no Ribonucleoproteins Aging-related enzymes Anti-aging substances DNA replication EC 2.7.7 Senescence Telomere-related proteins