|
Cryo-EM
Cryogenic electron microscopy (cryo-EM) is a cryomicroscopy technique applied on samples cooled to cryogenic temperatures. For biological specimens, the structure is preserved by embedding in an environment of vitreous ice. An aqueous sample solution is applied to a grid-mesh and plunge-frozen in liquid ethane or a mixture of liquid ethane and propane. While development of the technique began in the 1970s, recent advances in detector technology and software algorithms have allowed for the determination of biomolecular structures at near-atomic resolution. This has attracted wide attention to the approach as an alternative to X-ray crystallography or NMR spectroscopy for macromolecular structure determination without the need for crystallization. In 2017, the Nobel Prize in Chemistry was awarded to Jacques Dubochet, Joachim Frank, and Richard Henderson "for developing cryo-electron microscopy for the high-resolution structure determination of biomolecules in solution." '' Natu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmission Electron Cryomicroscopy
Transmission electron cryomicroscopy (CryoTEM), commonly known as cryo-EM, is a form of cryogenic electron microscopy, more specifically a type of transmission electron microscopy (TEM) where the sample is studied at cryogenic temperatures (generally liquid-nitrogen temperatures). Cryo-EM is gaining popularity in structural biology. The utility of transmission electron cryomicroscopy stems from the fact that it allows the observation of specimens that have not been stained or fixed in any way, showing them in their native environment. This is in contrast to X-ray crystallography, which requires crystallizing the specimen, which can be difficult, and placing them in non-physiological environments, which can occasionally lead to functionally irrelevant conformational changes. Advances in electron detector technology, particularly DED (Direct Electron Detectors) as well as more powerful software imaging algorithms have allowed for the determination of macromolecular structures at n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cryo-TEM
Transmission electron cryomicroscopy (CryoTEM), commonly known as cryo-EM, is a form of cryogenic electron microscopy, more specifically a type of transmission electron microscopy (TEM) where the sample is studied at cryogenic temperatures (generally liquid-nitrogen temperatures). Cryo-EM is gaining popularity in structural biology. The utility of transmission electron cryomicroscopy stems from the fact that it allows the observation of specimens that have not been stained or fixed in any way, showing them in their native environment. This is in contrast to X-ray crystallography, which requires crystallizing the specimen, which can be difficult, and placing them in non-physiological environments, which can occasionally lead to functionally irrelevant conformational changes. Advances in electron detector technology, particularly DED (Direct Electron Detectors) as well as more powerful software imaging algorithms have allowed for the determination of macromolecular structures at n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Crystallography
Electron crystallography is a method to determine the arrangement of atoms in solids using a transmission electron microscope (TEM). Comparison with X-ray crystallography It can complement X-ray crystallography for studies of very small crystals ( 1 micrometer) crystals impervious to electrons, which only penetrate short distances. One of the main difficulties in X-ray crystallography is determining phases in the diffraction pattern. Because of the complexity of X-ray lenses, it is difficult to form an image of the crystal being diffracted, and hence phase information is lost. Fortunately, electron microscopes can resolve atomic structure in real space and the crystallographic structure factor phase information can be experimentally determined from an image's Fourier transform. The Fourier transform of an atomic resolution image is similar, but different, to a diffraction pattern—with reciprocal lattice spots reflecting the symmetry and spacing of a crystal. Aaron Klug was t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jacques Dubochet
Jacques Dubochet (born 8 June 1942) is a retired Swiss biophysicist. He is a former researcher at the European Molecular Biology Laboratory in Heidelberg, Germany, and an honorary professor of biophysics at the University of Lausanne in Switzerland. In 2017, he received the Nobel Prize in Chemistry together with Joachim Frank and Richard Henderson "for developing cryo-electron microscopy for the high-resolution structure determination of biomolecules in solution". He received the Royal Photographic Society Progress Medal, alongside his colleagues Professor Joachim Frank and Dr Richard Henderson, in 2018 for 'an important advance in the scientific or technological development of photography or imaging in the widest sense'. Career Dubochet started to study physics at the ''École polytechnique de l' Université de Lausanne'' (now École polytechnique fédérale de Lausanne) in 1962 and obtained his degree in physical engineering in 1967. He obtained a Certificate of Molecular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Structural Biology
Structural biology is a field that is many centuries old which, and as defined by the Journal of Structural Biology, deals with structural analysis of living material (formed, composed of, and/or maintained and refined by living cells) at every level of organization. Early structural biologists throughout the 19th and early 20th centuries were primarily only able to study structures to the limit of the naked eye's visual acuity and through magnifying glasses and light microscopes. In the 20th century, a variety of experimental techniques were developed to examine the 3D structures of biological molecules. The most prominent techniques are X-ray crystallography, nuclear magnetic resonance, and electron microscopy. Through the discovery of X-rays and its applications to protein crystals, structural biology was revolutionized, as now scientists could obtain the three-dimensional structures of biological molecules in atomic detail. Likewise, NMR spectroscopy allowed information about p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carl Zeiss Elektronenmikroskop Marburg
Carl may refer to: * Carl, Georgia, city in USA * Carl, West Virginia, an unincorporated community *Carl (name), includes info about the name, variations of the name, and a list of people with the name * Carl², a TV series * "Carl", an episode of television series ''Aqua Teen Hunger Force'' * An informal nickname for a student or alum of Carleton College CARL may refer to: * Canadian Association of Research Libraries * Colorado Alliance of Research Libraries See also * Carle (other) * Charles *Carle, a surname * Karl (other) *Karle (other) Karle may refer to: Places * Karle (Svitavy District), a municipality and village in the Czech Republic * Karli, India, a town in Maharashtra, India ** Karla Caves, a complex of Buddhist cave shrines * Karle, Belgaum, a settlement in Belgaum di ... {{disambig ja:カール zh:卡尔 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid
Solid is one of the four fundamental states of matter (the others being liquid, gas, and plasma). The molecules in a solid are closely packed together and contain the least amount of kinetic energy. A solid is characterized by structural rigidity and resistance to a force applied to the surface. Unlike a liquid, a solid object does not flow to take on the shape of its container, nor does it expand to fill the entire available volume like a gas. The atoms in a solid are bound to each other, either in a regular geometric lattice (crystalline solids, which include metals and ordinary ice), or irregularly (an amorphous solid such as common window glass). Solids cannot be compressed with little pressure whereas gases can be compressed with little pressure because the molecules in a gas are loosely packed. The branch of physics that deals with solids is called solid-state physics, and is the main branch of condensed matter physics (which also includes liquids). Material ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
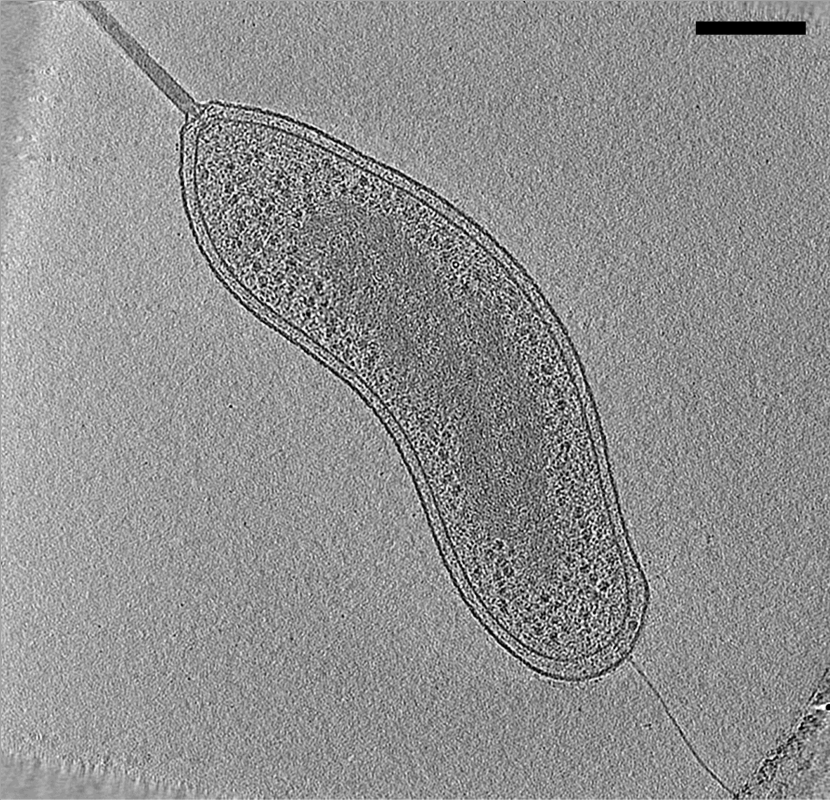
Electron Cryotomography
Electron cryotomography (CryoET) is an imaging technique used to produce high-resolution (~1–4 nm) three-dimensional views of samples, often (but not limited to) biological macromolecules and cells. CryoET is a specialized application of transmission electron cryomicroscopy (CryoTEM) in which samples are imaged as they are tilted, resulting in a series of 2D images that can be combined to produce a 3D reconstruction, similar to a CT scan of the human body. In contrast to other electron tomography techniques, samples are imaged under cryogenic conditions (< −150 °C). For cellular material, the structure is immobilized in non-crystalline, vitreous ice, allowing them to be imaged without dehydration or chemical fixation, which would otherwise disrupt or distort biological structures. Description of technique [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics, as if they were tennis balls for example, is not possible due to quantum effects. More than 99.94% of an atom's mass is in the nucleus. The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge. If the number of protons and electrons are equal, then the atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively – such atoms are called ions. The electrons of an ato ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A polypeptide is a longer, continuous, unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. A polypeptide that contains more than approximately 50 amino acids is known as a protein. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microcrystal Electron Diffraction
Microcrystal electron diffraction, or MicroED, is a Cryogenic electron microscopy, CryoEM method that was developed by the Tamir Gonen, Gonen laboratory in late 2013 at the Janelia Research Campus of the Howard Hughes Medical Institute. MicroED is a form of electron crystallography where thin 3D crystals are used for structure determination by electron diffraction. The method was developed for Protein structure, structure determination of proteins from nanocrystals that are typically not suitable for X-ray diffraction because of their size. Crystals that are one billionth the size needed for X-ray crystallography can yield high quality data. The samples are frozen hydrated as for all other CryoEM modalities but instead of using the Transmission electron microscopy, transmission electron microscope (Transmission electron cryomicroscopy, TEM) in imaging mode one uses it in diffraction mode with an extremely low electron exposure (typically < 0.01 e−/Å2/s). Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)