Staphylococcus aureus on:
[Wikipedia]
[Google]
[Amazon]

 ''Staphylococcus aureus'' is a
''Staphylococcus aureus'' is a
 ''Staphylococcus aureus'' (, Greek ,
''Staphylococcus aureus'' (, Greek ,
 While ''S. aureus'' usually acts as a commensal bacterium, asymptomatically colonizing about 30% of the human population, it can sometimes cause disease. In particular, ''S. aureus'' is one of the most common causes of
While ''S. aureus'' usually acts as a commensal bacterium, asymptomatically colonizing about 30% of the human population, it can sometimes cause disease. In particular, ''S. aureus'' is one of the most common causes of
 ;Staphylococcal pigments
Some strains of ''S. aureus'' are capable of producing staphyloxanthin – a golden-coloured carotenoid
;Staphylococcal pigments
Some strains of ''S. aureus'' are capable of producing staphyloxanthin – a golden-coloured carotenoid
 Depending upon the type of infection present, an appropriate specimen is obtained accordingly and sent to the laboratory for definitive identification by using biochemical or enzyme-based tests. A Gram stain is first performed to guide the way, which should show typical
Depending upon the type of infection present, an appropriate specimen is obtained accordingly and sent to the laboratory for definitive identification by using biochemical or enzyme-based tests. A Gram stain is first performed to guide the way, which should show typical
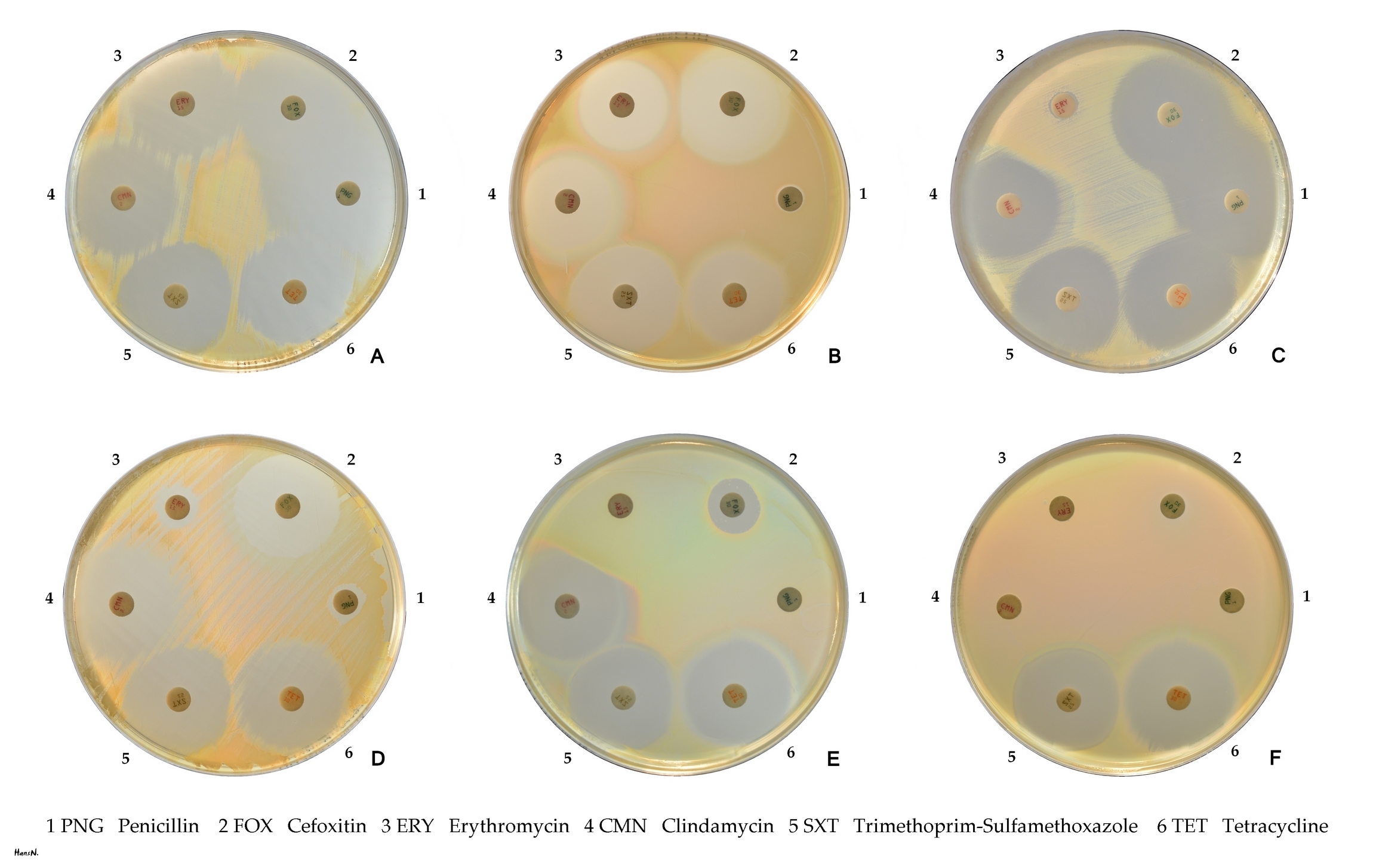 Resistance to methicillin is mediated via the ''mec'' operon, part of the staphylococcal cassette chromosome mec (SCC''mec''). SCCmec is a family of mobile genetic elements, which is a major driving force of ''S. aureus'' evolution. Resistance is conferred by the ''mecA'' gene, which codes for an altered penicillin-binding protein (PBP2a or PBP2') that has a lower affinity for binding β-lactams (penicillins, cephalosporins, and carbapenems). This allows for resistance to all β-lactam antibiotics, and obviates their clinical use during MRSA infections. Studies have explained that this mobile genetic element has been acquired by different lineages in separate gene transfer events, indicating that there is not a common ancestor of differing MRSA strains. One study suggests that MRSA sacrifices virulence, for example, toxin production and invasiveness, for survival and creation of biofilms
Aminoglycoside antibiotics, such as kanamycin, gentamicin,
Resistance to methicillin is mediated via the ''mec'' operon, part of the staphylococcal cassette chromosome mec (SCC''mec''). SCCmec is a family of mobile genetic elements, which is a major driving force of ''S. aureus'' evolution. Resistance is conferred by the ''mecA'' gene, which codes for an altered penicillin-binding protein (PBP2a or PBP2') that has a lower affinity for binding β-lactams (penicillins, cephalosporins, and carbapenems). This allows for resistance to all β-lactam antibiotics, and obviates their clinical use during MRSA infections. Studies have explained that this mobile genetic element has been acquired by different lineages in separate gene transfer events, indicating that there is not a common ancestor of differing MRSA strains. One study suggests that MRSA sacrifices virulence, for example, toxin production and invasiveness, for survival and creation of biofilms
Aminoglycoside antibiotics, such as kanamycin, gentamicin,
StopMRSANow.org
— Discusses how to prevent the spread of MRSA
TheMRSA.com
— Understand what the MRSA infection is all about. * *
Type strain of ''Staphylococcus aureus'' at Bac''Dive'' – the Bacterial Diversity Metadatabase
{{Authority control Food microbiology aureus Bacteriology Gram-positive bacteria Bacterial diseases Pathogenic bacteria Healthcare-associated infections Bacteria described in 1884

 ''Staphylococcus aureus'' is a
''Staphylococcus aureus'' is a Gram-positive
In bacteriology, gram-positive bacteria are bacteria that give a positive result in the Gram stain test, which is traditionally used to quickly classify bacteria into two broad categories according to their type of cell wall.
The Gram stain is ...
spherically shaped bacterium, a member of the Bacillota
The Bacillota (synonym Firmicutes) are a phylum of bacteria, most of which have Gram-positive cell wall structure. They have round cells, called cocci (singular coccus), or rod-like forms (bacillus). A few Bacillota, such as '' Megasphaera'', ...
, and is a usual member of the microbiota of the body, frequently found in the upper respiratory tract and on the skin. It is often positive for catalase and nitrate reduction and is a facultative anaerobe, meaning that it can grow without oxygen. Although ''S. aureus'' usually acts as a commensal of the human microbiota, it can also become an opportunistic pathogen, being a common cause of skin infections including abscesses, respiratory infections such as sinusitis, and food poisoning. Pathogenic strains often promote infection
An infection is the invasion of tissue (biology), tissues by pathogens, their multiplication, and the reaction of host (biology), host tissues to the infectious agent and the toxins they produce. An infectious disease, also known as a transmis ...
s by producing virulence factors such as potent protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
toxins, and the expression of a cell-surface protein that binds and inactivates antibodies. ''S. aureus'' is one of the leading pathogens for deaths associated with antimicrobial resistance and the emergence of antibiotic-resistant strains, such as methicillin-resistant ''S. aureus'' (MRSA). The bacterium is a worldwide problem in clinical medicine. Despite much research and development
Research and development (R&D or R+D), known in some countries as OKB, experiment and design, is the set of innovative activities undertaken by corporations or governments in developing new services or products. R&D constitutes the first stage ...
, no vaccine
A vaccine is a biological Dosage form, preparation that provides active acquired immunity to a particular infectious disease, infectious or cancer, malignant disease. The safety and effectiveness of vaccines has been widely studied and verifi ...
for ''S. aureus'' has been approved.
An estimated 21% to 30% of the human population are long-term carriers of ''S. aureus'', which can be found as part of the normal skin microbiota, in the nostrils, and as a normal inhabitant of the lower reproductive tract of females. ''S. aureus'' can cause a range of illnesses, from minor skin infections, such as pimples, impetigo, boils, cellulitis, folliculitis, carbuncles, scalded skin syndrome, and abscesses, to life-threatening diseases such as pneumonia, meningitis
Meningitis is acute or chronic inflammation of the protective membranes covering the brain and spinal cord, collectively called the meninges. The most common symptoms are fever, intense headache, vomiting and neck stiffness and occasion ...
, osteomyelitis
Osteomyelitis (OM) is the infectious inflammation of bone marrow. Symptoms may include pain in a specific bone with overlying redness, fever, and weakness. The feet, spine, and hips are the most commonly involved bones in adults.
The cause is ...
, endocarditis, toxic shock syndrome
Toxic shock syndrome (TSS) is a condition caused by Exotoxin, bacterial toxins. Symptoms may include fever, rash, skin peeling, and low blood pressure. There may also be symptoms related to the specific underlying infection such as mastitis, ...
, bacteremia
Bloodstream infections (BSIs) are infections of blood caused by blood-borne pathogens. The detection of microbes in the blood (most commonly accomplished by blood cultures) is always abnormal. A bloodstream infection is different from sepsis, wh ...
, and sepsis
Sepsis is a potentially life-threatening condition that arises when the body's response to infection causes injury to its own tissues and organs.
This initial stage of sepsis is followed by suppression of the immune system. Common signs and s ...
. It is still one of the five most common causes of hospital-acquired infections and is often the cause of wound infections following surgery
Surgery is a medical specialty that uses manual and instrumental techniques to diagnose or treat pathological conditions (e.g., trauma, disease, injury, malignancy), to alter bodily functions (e.g., malabsorption created by bariatric surgery s ...
. Each year, around 500,000 hospital patients in the United States contract a staphylococcal infection, chiefly by ''S. aureus''. Up to 50,000 deaths each year in the U.S. are linked to staphylococcal infection.
History
Discovery
In 1880, Alexander Ogston, a Scottish surgeon, discovered that ''Staphylococcus'' can cause wound infections after noticing groups of bacteria in pus from a surgical abscess during a procedure he was performing. He named it ''Staphylococcus'' after its clustered appearance evident under a microscope. Then, in 1884, German scientist Friedrich Julius Rosenbach identified ''Staphylococcus aureus'', discriminating and separating it from '' Staphylococcus albus'', a related bacterium. In the early 1930s, doctors began to use a more streamlined test to detect the presence of an ''S. aureus'' infection by the means of coagulase testing, which enables detection of an enzyme produced by the bacterium. Prior to the 1940s, ''S. aureus'' infections were fatal in the majority of patients. However, doctors discovered that the use of penicillin could cure ''S. aureus'' infections. Unfortunately, by the end of the 1940s, penicillin resistance became widespread amongst this bacterium population and outbreaks of the resistant strain began to occur.Evolution
''Staphylococcus aureus'' can be sorted into ten dominant human lineages. There are numerous minor lineages as well, but these are not seen in the population as often. Genomes of bacteria within the same lineage are mostly conserved, with the exception of mobile genetic elements. Mobile genetic elements that are common in ''S. aureus'' include bacteriophages, pathogenicity islands, plasmids, transposons, and staphylococcal cassette chromosomes. These elements have enabled ''S. aureus'' to continually evolve and gain new traits. There is a great deal of genetic variation within the ''S. aureus'' species''.'' A study by Fitzgerald et al. (2001) revealed that approximately 22% of the ''S. aureus'' genome is non-coding and thus can differ from bacterium to bacterium. An example of this difference is seen in the species' virulence. Only a few strains of ''S. aureus'' are associated with infections in humans. This demonstrates that there is a large range of infectious ability within the species. It has been proposed that one possible reason for the great deal of heterogeneity within the species could be due to its reliance on heterogeneous infections. This occurs when multiple different types of ''S. aureus'' cause an infection within a host. The different strains can secrete different enzymes or bring different antibiotic resistances to the group, increasing its pathogenic ability. Thus, there is a need for a large number of mutations and acquisitions of mobile genetic elements. Another notable evolutionary process within the ''S. aureus'' species is its co-evolution with its human hosts. Over time, this parasitic relationship has led to the bacterium's ability to be carried in the nasopharynx of humans without causing symptoms or infection. This allows it to be passed throughout the human population, increasing its fitness as a species. However, only approximately 50% of the human population are carriers of ''S. aureus'', with 20% as continuous carriers and 30% as intermittent. This leads scientists to believe that there are many factors that determine whether ''S. aureus'' is carried asymptomatically in humans, including factors that are specific to an individual person. According to a 1995 study by Hofman et al., these factors may include age, sex, diabetes, and smoking. They also determined some genetic variations in humans that lead to an increased ability for ''S. aureus'' to colonize, notably a polymorphism in the glucocorticoid receptor gene that results in largercorticosteroid
Corticosteroids are a class of steroid hormones that are produced in the adrenal cortex of vertebrates, as well as the synthetic analogues of these hormones. Two main classes of corticosteroids, glucocorticoids and mineralocorticoids, are invo ...
production. In conclusion, there is evidence that any strain of this bacterium can become invasive, as this is highly dependent upon human factors.
Though ''S. aureus'' has quick reproductive and micro-evolutionary rates, there are multiple barriers that prevent evolution with the species. One such barrier is AGR, which is a global accessory gene regulator within the bacteria. This such regulator has been linked to the virulence level of the bacteria. Loss of function mutations within this gene have been found to increase the fitness of the bacterium containing it. Thus, ''S. aureus'' must make a trade-off to increase their success as a species, exchanging reduced virulence for increased drug resistance. Another barrier to evolution is the Sau1 Type I restriction modification (RM) system. This system exists to protect the bacterium from foreign DNA by digesting it. Exchange of DNA between the same lineage is not blocked, since they have the same enzymes and the RM system does not recognize the new DNA as foreign, but transfer between different lineages is blocked.
Microbiology
 ''Staphylococcus aureus'' (, Greek ,
''Staphylococcus aureus'' (, Greek , Latin
Latin ( or ) is a classical language belonging to the Italic languages, Italic branch of the Indo-European languages. Latin was originally spoken by the Latins (Italic tribe), Latins in Latium (now known as Lazio), the lower Tiber area aroun ...
, ) is a facultative anaerobic, Gram-positive coccal (round) bacterium also known as "golden staph" and "oro staphira". ''S. aureus'' is nonmotile and does not form spores. In medical literature, the bacterium is often referred to as ''S. aureus'', ''Staph aureus'' or ''Staph a.''. ''S. aureus'' appears as staphylococci (grape-like clusters) when viewed through a microscope, and has large, round, golden-yellow colonies, often with hemolysis
Hemolysis or haemolysis (), also known by #Nomenclature, several other names, is the rupturing (lysis) of red blood cells (erythrocytes) and the release of their contents (cytoplasm) into surrounding fluid (e.g. blood plasma). Hemolysis may ...
, when grown on blood agar plates. ''S. aureus'' reproduces asexually by binary fission. Complete separation of the daughter cells is mediated by ''S. aureus'' autolysin, and in its absence or targeted inhibition, the daughter cells remain attached to one another and appear as clusters.
''Staphylococcus aureus'' is catalase-positive (meaning it can produce the enzyme catalase). Catalase converts hydrogen peroxide () to water and oxygen. Catalase-activity tests are sometimes used to distinguish staphylococci from enterococci and streptococci. Previously, ''S. aureus'' was differentiated from other staphylococci by the coagulase test. However, not all ''S. aureus'' strains are coagulase-positive and incorrect species identification can impact effective treatment and control measures.
Natural genetic transformation is a reproductive process involving DNA transfer from one bacterium to another through the intervening medium, and the integration of the donor sequence into the recipient genome by homologous recombination. ''S. aureus'' was found to be capable of natural genetic transformation, but only at low frequency under the experimental conditions employed. Further studies suggested that the development of competence for natural genetic transformation may be substantially higher under appropriate conditions, yet to be discovered.
Role in health
In humans, ''S. aureus'' can be present in the upper respiratory tract, gut mucosa, and skin as a member of the normal microbiota. However, because ''S. aureus'' can cause disease under certain host and environmental conditions, it is characterized as a pathobiont. In the United States, MRSA infections alone are estimated to cost the healthcare system over $3.2 billion annually. These infections account for nearly 20,000 deaths each year in the U.S., exceeding those caused by HIV/AIDS, Parkinson's disease, and homicide. Annually, over 119,000 bloodstream infections in the U.S. are attributed to ''S. aureus''. ''S. aureus'' infections are ranked as one of the costliest healthcare-associated infections (HAIs), with each case averaging $23,000 to $46,000 in treatment and hospital resource utilization. On average, patients with MRSA infections experience a lengthened hospital stay of approximately 6 to 11 days, which drives up inpatient care costs. The burden extends beyond direct healthcare expenses. Indirect costs, such as lost wages, reduced productivity, and long-term disability, can significantly amplify the overall economic toll. Severe ''S. aureus'' infections, including bacteremia, endocarditis, and osteomyelitis, often require prolonged recovery and rehabilitation, affecting patients' ability to return to work or perform daily activities. Hospitals also invest heavily in infection control protocols to limit the spread of ''S. aureus'', especially drug-resistant strains. These measures include routine screening, isolation practices, use of personal protective equipment, and antibiotic stewardship programs, which collectively contribute to rising operational costs. These necessary preventative measures can raise hospital costs by tens of thousands of dollars.Role in disease
 While ''S. aureus'' usually acts as a commensal bacterium, asymptomatically colonizing about 30% of the human population, it can sometimes cause disease. In particular, ''S. aureus'' is one of the most common causes of
While ''S. aureus'' usually acts as a commensal bacterium, asymptomatically colonizing about 30% of the human population, it can sometimes cause disease. In particular, ''S. aureus'' is one of the most common causes of bacteremia
Bloodstream infections (BSIs) are infections of blood caused by blood-borne pathogens. The detection of microbes in the blood (most commonly accomplished by blood cultures) is always abnormal. A bloodstream infection is different from sepsis, wh ...
and infective endocarditis. Additionally, it can cause various skin and soft-tissue infections, particularly when skin or mucosal barriers have been breached.
''Staphylococcus aureus'' infections can spread through contact with pus from an infected wound, skin-to-skin contact with an infected person, and contact with objects used by an infected person such as towels, sheets, clothing, or athletic equipment. Joint replacements put a person at particular risk of septic arthritis, staphylococcal endocarditis (infection of the heart valves), and pneumonia.
''Staphylococcus aureus'' is a significant cause of chronic biofilm infections on medical implants, and the repressor of toxins is part of the infection pathway.
''Staphylococcus aureus'' can lie dormant in the body for years undetected. Once symptoms begin to show, the host is contagious for another two weeks, and the overall illness lasts a few weeks. If untreated, though, the disease can be deadly. Deeply penetrating ''S. aureus'' infections can be severe.
Skin infections
Skin infections are the most common form of ''S. aureus'' infection. This can manifest in various ways, including small benign boils, folliculitis, impetigo, cellulitis, and more severe, invasive soft-tissue infections. ''Staphylococcus aureus'' is extremely prevalent in persons with atopic dermatitis (AD), more commonly known as eczema. It is mostly found in fertile, active places, including the armpits, hair, and scalp. Large pimples that appear in those areas may exacerbate the infection if lacerated. Colonization of ''S. aureus'' drives inflammation of AD. ''S. aureus'' is believed to exploit defects in the skin barrier of persons with atopic dermatitis, triggering cytokine expression and therefore exacerbating symptoms. This can lead to staphylococcal scalded skin syndrome, a severe form of which can be seen in newborns. The role of ''S. aureus'' in causing itching in atopic dermatitis has been studied. Antibiotics are commonly used to target overgrowth of ''S. aureus'' but their benefit is limited and they increase the risk of antimicrobial resistance. For these reasons, they are only recommended for people who not only present symptoms on the skin but feel systematically unwell.Food poisoning
''Staphylococcus aureus'' is also responsible for food poisoning and achieves this by generating toxins in the food, which is then ingested. Its incubation period lasts 30 minutes to eight hours, with the illness itself lasting from 30 minutes to 3 days. Preventive measures one can take to help prevent the spread of the disease include washing hands thoroughly with soap and water before preparing food. TheCenters for Disease Control and Prevention
The Centers for Disease Control and Prevention (CDC) is the National public health institutes, national public health agency of the United States. It is a Federal agencies of the United States, United States federal agency under the United S ...
recommends staying away from any food if ill, and wearing gloves if any open wounds occur on hands or wrists while preparing food. If storing food for longer than 2 hours, it is recommended to keep the food below 4.4 or above 60 °C (below 40 or above 140 °F).
Bone and joint infections
''Staphylococcus aureus'' is a common cause of major bone and joint infections, includingosteomyelitis
Osteomyelitis (OM) is the infectious inflammation of bone marrow. Symptoms may include pain in a specific bone with overlying redness, fever, and weakness. The feet, spine, and hips are the most commonly involved bones in adults.
The cause is ...
, septic arthritis, and infections following joint replacement surgeries.
Bacteremia
''Staphylococcus aureus'' is a leading cause of bloodstream infections throughout much of the industrialized world. Infection is generally associated with breaks in the skin or mucosal membranes due to surgery, injury, or use of intravascular devices such ascannula
A cannula (; Latin meaning 'little reed'; : cannulae or cannulas) is a tube that can be inserted into the body, often for the delivery or removal of fluid or for the gathering of samples. In simple terms, a cannula can surround the inner or out ...
s, hemodialysis machines, or hypodermic needles. Once the bacteria have entered the bloodstream, they can infect various organs, causing infective endocarditis, septic arthritis, and osteomyelitis
Osteomyelitis (OM) is the infectious inflammation of bone marrow. Symptoms may include pain in a specific bone with overlying redness, fever, and weakness. The feet, spine, and hips are the most commonly involved bones in adults.
The cause is ...
. This disease is particularly prevalent and severe in the very young and very old.
Without antibiotic treatment, ''S. aureus'' bacteremia has a case fatality rate around 80%. With antibiotic treatment, case fatality rates range from 15% to 50% depending on the age and health of the patient, as well as the antibiotic resistance of the ''S. aureus'' strain.
Medical implant infections
''Staphylococcus aureus'' is often found inbiofilm
A biofilm is a Syntrophy, syntrophic Microbial consortium, community of microorganisms in which cell (biology), cells cell adhesion, stick to each other and often also to a surface. These adherent cells become embedded within a slimy ext ...
s formed on medical devices implanted in the body or on human tissue. It is commonly found with another pathogen, '' Candida albicans'', forming multispecies biofilms. The latter is suspected to help ''S. aureus'' penetrate human tissue. A higher mortality is linked with multispecies biofilms.
''Staphylococcus aureus'' biofilm is the predominant cause of orthopedic implant-related infections, but is also found on cardiac implants, vascular grafts, various catheter
In medicine, a catheter ( ) is a thin tubing (material), tube made from medical grade materials serving a broad range of functions. Catheters are medical devices that can be inserted in the body to treat diseases or perform a surgical procedure. ...
s, and cosmetic surgical implants. After implantation, the surface of these devices becomes coated with host proteins, which provide a rich surface for bacterial attachment and biofilm formation. Once the device becomes infected, it must be completely removed, since ''S. aureus'' biofilm cannot be destroyed by antibiotic treatments.
Current therapy for ''S. aureus'' biofilm-mediated infections involves surgical removal of the infected device followed by antibiotic treatment. Conventional antibiotic treatment alone is not effective in eradicating such infections. An alternative to postsurgical antibiotic treatment is using antibiotic-loaded, dissolvable calcium sulfate beads, which are implanted with the medical device. These beads can release high doses of antibiotics at the desired site to prevent the initial infection.
Novel treatments for ''S. aureus'' biofilm involving nano silver particles, bacteriophages, and plant-derived antibiotic agents are being studied. These agents have shown inhibitory effects against ''S. aureus'' embedded in biofilms. A class of enzymes have been found to have biofilm matrix-degrading ability, thus may be used as biofilm dispersal agents in combination with antibiotics.
Animal infections
''Staphylococcus aureus'' can survive on dogs, cats, and horses, and can cause bumblefoot in chickens. Some believe health-care workers' dogs should be considered a significant source of antibiotic-resistant ''S. aureus'', especially in times of outbreak. In a 2008 study by Boost, O'Donoghue, and James, it was found that just about 90% of ''S. aureus'' colonized within pet dogs presented as resistant to at least one antibiotic. The nasal region has been implicated as the most important site of transfer between dogs and humans. ''Staphylococcus aureus'' is one of the causal agents of mastitis in dairy cows. Its largepolysaccharide
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long-chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with wat ...
capsule protects the organism from recognition by the cow's immune defenses.
Virulence factors
Enzymes
''Staphylococcus aureus'' produces various enzymes such as coagulase (bound and free coagulases) which facilitates the conversion of fibrinogen to fibrin to cause clots which is important in skin infections. Hyaluronidase (also known as spreading factor) breaks down hyaluronic acid and helps in spreading it. Deoxyribonuclease, which breaks down the DNA, protects ''S. aureus'' from neutrophil extracellular trap-mediated killing. ''S. aureus'' also produces lipase to digest lipids, staphylokinase to dissolve fibrin and aid in spread, and beta-lactamase for drug resistance.Toxins
Depending on the strain, ''S. aureus'' is capable of secreting several exotoxins, which can be categorized into three groups. Many of these toxins are associated with specific diseases. ;Superantigens : Antigens known as superantigens can inducetoxic shock syndrome
Toxic shock syndrome (TSS) is a condition caused by Exotoxin, bacterial toxins. Symptoms may include fever, rash, skin peeling, and low blood pressure. There may also be symptoms related to the specific underlying infection such as mastitis, ...
(TSS). This group comprises 25 staphylococcal enterotoxins (SEs) which have been identified to date and named alphabetically (SEA–SEZ), including enterotoxin type B as well as the toxic shock syndrome toxin TSST-1 which causes TSS associated with tampon use. Toxic shock syndrome is characterized by fever
Fever or pyrexia in humans is a symptom of an anti-infection defense mechanism that appears with Human body temperature, body temperature exceeding the normal range caused by an increase in the body's temperature Human body temperature#Fever, s ...
, erythematous rash, low blood pressure, shock, multiple organ failure, and skin peeling. Lack of antibody to TSST-1 plays a part in the pathogenesis of TSS. Other strains of ''S. aureus'' can produce an enterotoxin that is the causative agent of a type of gastroenteritis
Gastroenteritis, also known as infectious diarrhea, is an inflammation of the Human gastrointestinal tract, gastrointestinal tract including the stomach and intestine. Symptoms may include diarrhea, vomiting, and abdominal pain. Fever, lack of ...
. This form of gastroenteritis is self-limiting, characterized by vomiting and diarrhea 1–6 hours after ingestion of the toxin, with recovery in 8 to 24 hours. Symptoms include nausea, vomiting, diarrhea, and major abdominal pain.
;Exfoliative toxins
: Exfoliative toxins are exotoxins implicated in the disease staphylococcal scalded skin syndrome (SSSS), which occurs most commonly in infants and young children. It also may occur as epidemics in hospital nurseries. The protease activity of the exfoliative toxins causes peeling of the skin observed with SSSS.
;Other toxins
: Staphylococcal toxins that act on cell membranes include alpha toxin, beta toxin, delta toxin, and several bicomponent toxins. Strains of ''S. aureus'' can host phages, such as the prophage Φ-PVL that produces Panton-Valentine leukocidin (PVL), to increase virulence. The bicomponent toxin PVL is associated with severe necrotizing pneumonia in children. The genes encoding the components of PVL are encoded on a bacteriophage found in community-associated MRSA strains.
Type VII secretion system
A secretion system is a highly specialised multi-protein unit that is embedded in the cell envelope with the function of translocating effector proteins from inside of the cell to the extracellular space or into a target host cytosol. The exact structure and function of T7SS is yet to be fully elucidated. Currently, four proteins are known components of ''S. aureus'' type VII secretion system; EssC is a large integral membrane ATPase – which most likely powers the secretion systems and has been hypothesised forming part of the translocation channel. The other proteins are EsaA, EssB, EssA, that are membrane proteins that function alongside EssC to mediate protein secretion. The exact mechanism of how substrates reach the cell surface is unknown, as is the interaction of the three membrane proteins with each other and EssC. T7 dependent effector proteins EsaD is DNAendonuclease
In molecular biology, endonucleases are enzymes that cleave the phosphodiester bond within a polynucleotide chain (namely DNA or RNA). Some, such as deoxyribonuclease I, cut DNA relatively nonspecifically (with regard to sequence), while man ...
toxin secreted by ''S. aureus'', has been shown to inhibit growth of competitor ''S. aureus'' strain ''in vitro''. EsaD is cosecreted with chaperone EsaE, which stabilises EsaD structure and brings EsaD to EssC for secretion. Strains that produce EsaD also co-produce EsaG, a cytoplasmic anti-toxin that protects the producer strain from EsaD's toxicity.
TspA is another toxin that mediates intraspecies competition. It is a bacteriostatic toxin that has a membrane depolarising activity facilitated by its C-terminal domain. Tsai is a transmembrane protein that confers immunity to the producer strain of TspA, as well as the attacked strains. There is genetic variability of the C-terminal domain of TspA therefore, it seems like the strains may produce different TspA variants to increase competitiveness.
Toxins that play a role in intraspecies competition confers an advantage by promoting successful colonisation in polymicrobial communities such as the nasopharynx and lung by outcompeting lesser strains.
There are also T7 effector proteins that play role a in pathogenesis, for example mutational studies of ''S. aureus'' have suggested that EsxB and EsxC contribute to persistent infection in a murine abscess model.
EsxX has been implicated in neutrophil lysis, therefore suggested as contributing to the evasion of host immune system. Deletion of ''essX'' in ''S. aureus'' resulted in significantly reduced resistance to neutrophils and reduced virulence in murine skin and blood infection models.
Altogether, T7SS and known secreted effector proteins are a strategy of pathogenesis by improving fitness against competitor ''S. aureus'' species as well as increased virulence via evading the innate immune system and optimising persistent infections.
Small RNA
The list of small RNAs involved in the control of bacterial virulence in ''S. aureus'' is growing. This can be facilitated by factors such as increased biofilm formation in the presence of increased levels of such small RNAs. For example, RNAIII, SprD, SprC, RsaE, SprA1, SSR42, ArtR, SprX, Teg49, and IsrR.DNA repair
Host neutrophils cause DNA double-strand breaks in ''S. aureus'' through the production of reactive oxygen species. For infection of a host to be successful, ''S. aureus'' must survive such damages caused by the hosts' defenses. The two protein complex RexAB encoded by ''S. aureus'' is employed in the recombinational repair of DNA double-strand breaks.Strategies for post-transcriptional regulation by 3'untranslated region
Many mRNAs in ''S. aureus'' carry three prime untranslated regions (3'UTR) longer than 100nucleotide
Nucleotides are Organic compound, organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both o ...
s, which may potentially have a regulatory function.
Further investigation of i''caR'' mRNA (mRNA coding for the repressor of the main expolysaccharidic compound of the bacteria biofilm matrix) demonstrated that the 3'UTR binding to the 5' UTR
The 5′ untranslated region (also known as 5′ UTR, leader sequence, transcript leader, or leader RNA) is the region of a messenger RNA (mRNA) that is directly Upstream and downstream (DNA), upstream from the initiation codon. This region is im ...
can interfere with the translation initiation complex and generate a double stranded substrate for RNase III. The interaction is between the UCCCCUG motif in the 3'UTR and the Shine-Dalagarno region at the 5'UTR. Deletion of the motif resulted in IcaR repressor accumulation and inhibition of biofilm development. The biofilm formation is the main cause of ''Staphylococcus'' implant infections.
Biofilm
Biofilm
A biofilm is a Syntrophy, syntrophic Microbial consortium, community of microorganisms in which cell (biology), cells cell adhesion, stick to each other and often also to a surface. These adherent cells become embedded within a slimy ext ...
s are groups of microorganisms, such as bacteria, that attach to each other and grow on wet surfaces.Vidyasagar, A. (2016). What Are Biofilms? ''Live Science.'' The ''S. aureus'' biofilm is embedded in a glycocalyx slime layer and can consist of teichoic acids, host proteins, extracellular DNA (eDNA) and sometimes polysaccharide intercellular antigen (PIA). S. aureus biofilms are important in disease pathogenesis, as they can contribute to antibiotic resistance and immune system evasion. ''S. aureus'' biofilm has high resistance to antibiotic treatments and host immune response. One hypothesis for explaining this is that the biofilm matrix protects the embedded cells by acting as a barrier to prevent antibiotic penetration. However, the biofilm matrix is composed with many water channels, so this hypothesis is becoming increasingly less likely, but a biofilm matrix possibly contains antibiotic‐degrading enzymes such as β-lactamases, which can prevent antibiotic penetration. Another hypothesis is that the conditions in the biofilm matrix favor the formation of persister cells, which are highly antibiotic-resistant, dormant bacterial cells. ''S. aureus'' biofilms also have high resistance to host immune response. Though the exact mechanism of resistance is unknown, ''S. aureus'' biofilms have increased growth under the presence of cytokines produced by the host immune response. Host antibodies are less effective for ''S. aureus'' biofilm due to the heterogeneous antigen distribution, where an antigen may be present in some areas of the biofilm, but completely absent from other areas.
Studies in biofilm development have shown to be related to changes in gene expression. There are specific genes that were found to be crucial in the different biofilm growth stages. Two of these genes include rocD and gudB, which encode for the enzyme's ornithine-oxo-acid transaminase and glutamate dehydrogenase, which are important for amino acid metabolism. Studies have shown biofilm development rely on amino acids glutamine and glutamate
Glutamic acid (symbol Glu or E; known as glutamate in its anionic form) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a Essential amino acid, non-essential nutrient for humans, meaning that ...
for proper metabolic functions.
Other immunoevasive strategies
;Protein A Protein A is anchored to staphylococcal peptidoglycan pentaglycine bridges (chains of fiveglycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (G ...
residues) by the transpeptidase sortase A. Protein A, an IgG-binding protein, binds to the Fc region of an antibody
An antibody (Ab) or immunoglobulin (Ig) is a large, Y-shaped protein belonging to the immunoglobulin superfamily which is used by the immune system to identify and neutralize antigens such as pathogenic bacteria, bacteria and viruses, includin ...
. In fact, studies involving mutation of genes coding for protein A resulted in a lowered virulence of ''S. aureus'' as measured by survival in blood, which has led to speculation that protein A-contributed virulence requires binding of antibody Fc regions.
Protein A in various recombinant forms has been used for decades to bind and purify a wide range of antibodies by immunoaffinity chromatography. Transpeptidases, such as the sortases responsible for anchoring factors like protein A to the staphylococcal peptidoglycan, are being studied in hopes of developing new antibiotics to target MRSA infections.
 ;Staphylococcal pigments
Some strains of ''S. aureus'' are capable of producing staphyloxanthin – a golden-coloured carotenoid
;Staphylococcal pigments
Some strains of ''S. aureus'' are capable of producing staphyloxanthin – a golden-coloured carotenoid pigment
A pigment is a powder used to add or alter color or change visual appearance. Pigments are completely or nearly solubility, insoluble and reactivity (chemistry), chemically unreactive in water or another medium; in contrast, dyes are colored sub ...
. This pigment acts as a virulence factor, primarily by being a bacterial antioxidant which helps the microbe evade the reactive oxygen species which the host immune system uses to kill pathogens.
Mutant strains of ''S. aureus'' modified to lack staphyloxanthin are less likely to survive incubation with an oxidizing chemical, such as hydrogen peroxide, than pigmented strains. Mutant colonies are quickly killed when exposed to human neutrophils, while many of the pigmented colonies survive. In mice, the pigmented strains cause lingering abscesses when inoculated into wounds, whereas wounds infected with the unpigmented strains quickly heal.
These tests suggest the ''Staphylococcus'' strains use staphyloxanthin as a defence against the normal human immune system. Drugs designed to inhibit the production of staphyloxanthin may weaken the bacterium and renew its susceptibility to antibiotics. In fact, because of similarities in the pathways for biosynthesis of staphyloxanthin and human cholesterol
Cholesterol is the principal sterol of all higher animals, distributed in body Tissue (biology), tissues, especially the brain and spinal cord, and in Animal fat, animal fats and oils.
Cholesterol is biosynthesis, biosynthesized by all anima ...
, a drug developed in the context of cholesterol-lowering therapy was shown to block ''S. aureus'' pigmentation and disease progression in a mouse infection model.
;Resistance to Hypothiocyanous Acid (HOSCN)
''Staphylococcus aureus'' has developed an adaptive mechanism to tolerate hypothiocyanous acid (HOSCN), a potent oxidant produced by the human immune system. Compared to other methicillin-resistant ''S. aureus'' ( MRSA) strains and bacterial pathogens such as '' Pseudomonas aeruginosa'', ''Escherichia coli'', and '' Streptococcus pneumoniae'', ''S. aureus'' exhibits greater resistance to HOSCN.
This resistance is linked to the ''merA'' gene, which encodes a flavoprotein disulfide reductase (FDR) enzyme. ''S. aureus'' MerA shares similarities with HOSCN reductases from other bacteria, including ''S. pneumoniae'' (50% sequence identity, 66% positives) and RclA in '' E. coli'' (50% sequence identity, 65% positives). These enzymes play a crucial role in oxidative stress defense by using NADPH as a cofactor to reduce disulfide bonds, thereby mitigating the oxidative damage caused by HOSCN. This mechanism enhances ''S. aureus'' survival within the host by counteracting the immune system’s oxidative attack.
Functional characterization of MerA has revealed that the amino acid residue Cys43 (C43) is essential for its enzymatic activity against HOSCN. Additionally, the expression of ''merA'' in ''S. aureus'' is regulated by the ''hypR'' gene, a transcriptional suppressor that modulates the bacterial response to oxidative stress.
Classical diagnosis
Gram-positive
In bacteriology, gram-positive bacteria are bacteria that give a positive result in the Gram stain test, which is traditionally used to quickly classify bacteria into two broad categories according to their type of cell wall.
The Gram stain is ...
bacteria, cocci, in clusters. Second, the isolate is cultured on mannitol salt agar, which is a selective medium with 7.5% NaCl that allows ''S. aureus'' to grow, producing yellow-colored colonies as a result of mannitol fermentation and subsequent drop in the medium's pH.
Furthermore, for differentiation on the species level, catalase (positive for all ''Staphylococcus'' species), coagulase ( fibrin clot formation, positive for ''S. aureus''), DNAse (zone of clearance on DNase agar), lipase (a yellow color and rancid odor smell), and phosphatase (a pink color) tests are all done. For staphylococcal food poisoning, phage typing can be performed to determine whether the staphylococci recovered from the food were the source of infection.
Rapid diagnosis and typing
Diagnostic microbiology laboratories and reference laboratories are key for identifying outbreaks and new strains of ''S. aureus''. Recent genetic advances have enabled reliable and rapid techniques for the identification and characterization of clinical isolates of ''S. aureus'' in real time. These tools support infection control strategies to limit bacterial spread and ensure the appropriate use of antibiotics. Quantitative PCR is increasingly being used to identify outbreaks of infection. When observing the evolvement of ''S. aureus'' and its ability to adapt to each modified antibiotic, two basic methods known as "band-based" or "sequence-based" are employed. Keeping these two methods in mind, other methods such as multilocus sequence typing (MLST), pulsed-field gel electrophoresis (PFGE), bacteriophage typing, spa locus typing, and SCCmec typing are often conducted more than others. With these methods, it can be determined where strains of MRSA originated and also where they are currently. With MLST, this technique of typing uses fragments of several housekeeping genes known as ''aroE, glpF, gmk, pta, tip,'' and ''yqiL''. These sequences are then assigned a number which give to a string of several numbers that serve as the allelic profile. Although this is a common method, a limitation about this method is the maintenance of the microarray which detects newly allelic profiles, making it a costly and time-consuming experiment. With PFGE, a method which is still very much used dating back to its first success in 1980s, remains capable of helping differentiate MRSA isolates. To accomplish this, the technique uses multiple gel electrophoresis, along with a voltage gradient to display clear resolutions of molecules. The ''S. aureus'' fragments then transition down the gel, producing specific band patterns that are later compared with other isolates in hopes of identifying related strains. Limitations of the method include practical difficulties with uniform band patterns and PFGE sensitivity as a whole. Spa locus typing is also considered a popular technique that uses a single locus zone in a polymorphic region of ''S. aureus'' to distinguish any form of mutations. Although this technique is often inexpensive and less time-consuming, the chance of losing discriminatory power making it hard to differentiate between MLST clonal complexes exemplifies a crucial limitation.Treatment
For susceptible strains, the treatment of choice for ''S. aureus'' infection ispenicillin
Penicillins (P, PCN or PEN) are a group of beta-lactam antibiotic, β-lactam antibiotics originally obtained from ''Penicillium'' Mold (fungus), moulds, principally ''Penicillium chrysogenum, P. chrysogenum'' and ''Penicillium rubens, P. ru ...
. An antibiotic derived from some ''Penicillium
''Penicillium'' () is a genus of Ascomycota, ascomycetous fungus, fungi that is part of the mycobiome of many species and is of major importance in the natural environment, in food spoilage, and in food and drug production.
Some members of th ...
'' fungal species, penicillin inhibits the formation of peptidoglycan cross-linkages that provide the rigidity and strength in a bacterial cell wall. The four-membered β-lactam ring of penicillin is bound to enzyme DD-transpeptidase, an enzyme that when functional, cross-links chains of peptidoglycan that form bacterial cell walls. The binding of β-lactam to DD-transpeptidase inhibits the enzyme's functionality and it can no longer catalyze the formation of the cross-links. As a result, cell wall formation and degradation are imbalanced, thus resulting in cell death. In most countries, however, penicillin resistance is extremely common (>90%), and first-line therapy is most commonly a penicillinase-resistant β-lactam antibiotic (for example, oxacillin or flucloxacillin, both of which have the same mechanism of action as penicillin) or vancomycin, depending on local resistance patterns. Combination therapy with gentamicin may be used to treat serious infections, such as endocarditis, but its use is controversial because of the high risk of damage to the kidneys. The duration of treatment depends on the site of infection and on severity. Adjunctive rifampicin has been historically used in the management of ''S aureus'' bacteraemia, but randomised controlled trial evidence has shown this to be of no overall benefit over standard antibiotic therapy.
Antibiotic resistance in ''S. aureus'' was uncommon when penicillin was first introduced in 1943. Indeed, the original Petri dish on which Alexander Fleming of Imperial College London observed the antibacterial activity of the ''Penicillium
''Penicillium'' () is a genus of Ascomycota, ascomycetous fungus, fungi that is part of the mycobiome of many species and is of major importance in the natural environment, in food spoilage, and in food and drug production.
Some members of th ...
'' fungus was growing a culture of ''S. aureus''. By 1950, 40% of hospital ''S. aureus'' isolates were penicillin-resistant; by 1960, this had risen to 80%.
Methicillin-resistant Staphylococcus aureus (MRSA, often pronounced or ), is one of a number of greatly feared strains of ''S. aureus'' which have become resistant to most β-lactam antibiotics. For this reason, vancomycin, a glycopeptide antibiotic, is commonly used to combat MRSA. Vancomycin inhibits the synthesis of peptidoglycan, but unlike β-lactam antibiotics, glycopeptide antibiotics target and bind to amino acids in the cell wall, preventing peptidoglycan cross-linkages from forming. MRSA strains are most often found associated with institutions such as hospitals, but are becoming increasingly prevalent in community-acquired infections.
Minor skin infections can be treated with triple antibiotic ointment. One topical agent that is prescribed is mupirocin, a protein synthesis inhibitor that is produced naturally by Pseudomonas fluorescens and has seen success for treatment of S. aureus nasal carriage.
Antibiotic resistance
''Staphylococcus aureus'' was found to be the second leading pathogen for deaths associated with antimicrobial resistance in 2019. Staphylococcal resistance to penicillin is mediated by penicillinase (a form of beta-lactamase) production: an enzyme that cleaves the β-lactam ring of the penicillin molecule, rendering the antibiotic ineffective. Penicillinase-resistant β-lactam antibiotics, such as methicillin, nafcillin, oxacillin, cloxacillin, dicloxacillin, and flucloxacillin are able to resist degradation by staphylococcal penicillinase. Resistance to methicillin is mediated via the ''mec'' operon, part of the staphylococcal cassette chromosome mec (SCC''mec''). SCCmec is a family of mobile genetic elements, which is a major driving force of ''S. aureus'' evolution. Resistance is conferred by the ''mecA'' gene, which codes for an altered penicillin-binding protein (PBP2a or PBP2') that has a lower affinity for binding β-lactams (penicillins, cephalosporins, and carbapenems). This allows for resistance to all β-lactam antibiotics, and obviates their clinical use during MRSA infections. Studies have explained that this mobile genetic element has been acquired by different lineages in separate gene transfer events, indicating that there is not a common ancestor of differing MRSA strains. One study suggests that MRSA sacrifices virulence, for example, toxin production and invasiveness, for survival and creation of biofilms
Aminoglycoside antibiotics, such as kanamycin, gentamicin,
Resistance to methicillin is mediated via the ''mec'' operon, part of the staphylococcal cassette chromosome mec (SCC''mec''). SCCmec is a family of mobile genetic elements, which is a major driving force of ''S. aureus'' evolution. Resistance is conferred by the ''mecA'' gene, which codes for an altered penicillin-binding protein (PBP2a or PBP2') that has a lower affinity for binding β-lactams (penicillins, cephalosporins, and carbapenems). This allows for resistance to all β-lactam antibiotics, and obviates their clinical use during MRSA infections. Studies have explained that this mobile genetic element has been acquired by different lineages in separate gene transfer events, indicating that there is not a common ancestor of differing MRSA strains. One study suggests that MRSA sacrifices virulence, for example, toxin production and invasiveness, for survival and creation of biofilms
Aminoglycoside antibiotics, such as kanamycin, gentamicin, streptomycin
Streptomycin is an antibiotic medication used to treat a number of bacterial infections, including tuberculosis, Mycobacterium avium complex, ''Mycobacterium avium'' complex, endocarditis, brucellosis, Burkholderia infection, ''Burkholderia'' i ...
, were once effective against staphylococcal infections until strains evolved mechanisms to inhibit the aminoglycosides' action, which occurs via protonated amine and/or hydroxyl interactions with the ribosomal RNA
Ribosomal ribonucleic acid (rRNA) is a type of non-coding RNA which is the primary component of ribosomes, essential to all cells. rRNA is a ribozyme which carries out protein synthesis in ribosomes. Ribosomal RNA is transcribed from ribosomal ...
of the bacterial 30S ribosomal subunit. Three main mechanisms of aminoglycoside resistance mechanisms are currently and widely accepted: aminoglycoside modifying enzymes, ribosomal mutations, and active efflux of the drug out of the bacteria.
Aminoglycoside-modifying enzymes inactivate the aminoglycoside by covalently attaching either a phosphate
Phosphates are the naturally occurring form of the element phosphorus.
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthop ...
, nucleotide
Nucleotides are Organic compound, organic molecules composed of a nitrogenous base, a pentose sugar and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both o ...
, or acetyl moiety to either the amine or the alcohol key functional group (or both groups) of the antibiotic. This changes the charge or sterically hinders the antibiotic, decreasing its ribosomal binding affinity. In ''S. aureus'', the best-characterized aminoglycoside-modifying enzyme is aminoglycoside adenylyltransferase 4' IA (''ANT(4')IA''). This enzyme has been solved by X-ray crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring th ...
. The enzyme is able to attach an adenyl moiety to the 4' hydroxyl group of many aminoglycosides, including kanamycin and gentamicin.
Glycopeptide resistance is typically mediated by acquisition of the ''vanA'' gene, which originates from the Tn1546 transposon found in a plasmid in enterococci and codes for an enzyme that produces an alternative peptidoglycan to which vancomycin will not bind.
Today, ''S. aureus'' has become resistant to many commonly used antibiotics. In the UK, only 2% of all ''S. aureus'' isolates are sensitive to penicillin, with a similar picture in the rest of the world. The β-lactamase-resistant penicillins (methicillin, oxacillin, cloxacillin, and flucloxacillin) were developed to treat penicillin-resistant ''S. aureus'', and are still used as first-line treatment. Methicillin was the first antibiotic in this class to be used (it was introduced in 1959), but only two years later, the first case of methicillin-resistant ''Staphylococcus aureus'' (MRSA) was reported in England.
Despite this, MRSA generally remained an uncommon finding, even in hospital settings, until the 1990s, when the MRSA prevalence in hospitals exploded, and it is now endemic
Endemism is the state of a species being found only in a single defined geographic location, such as an island, state, nation, country or other defined zone; organisms that are indigenous to a place are not endemic to it if they are also foun ...
. Now, methicillin-resistant ''Staphylococcus aureus'' (MRSA) is not only a human pathogen causing a variety of infections, such as skin and soft tissue infection (SSTI), pneumonia, and sepsis, but it also can cause disease in animals, known as livestock-associated MRSA (LA-MRSA).
MRSA infections in both the hospital and community setting are commonly treated with non-β-lactam antibiotics, such as clindamycin (a lincosamine) and co-trimoxazole (also commonly known as trimethoprim/ sulfamethoxazole). Resistance to these antibiotics has also led to the use of new, broad-spectrum anti-Gram-positive antibiotics, such as linezolid, because of its availability as an oral drug. First-line treatment for serious invasive infections due to MRSA is currently glycopeptide antibiotics (vancomycin and teicoplanin). A number of problems with these antibiotics occur, such as the need for intravenous administration (no oral preparation is available), toxicity, and the need to monitor drug levels regularly by blood tests. Also, glycopeptide antibiotics do not penetrate very well into infected tissues (this is a particular concern with infections of the brain and meninges and in endocarditis). Glycopeptides must not be used to treat methicillin-sensitive ''S. aureus'' (MSSA), as outcomes are inferior.
Daptomycin is a cyclic lipopeptide antibiotic primarily used for treating Gram-positive bacterial infections, including those caused by Staphylococcus aureus. It was first approved in 2003 and is especially effective against resistant strains like methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Staphylococcus aureus (VRSA).
Daptomycin works in a unique way compared to other antibiotics. It including calcium-dependent membrane binding, disruption of membrane potentia and bacterial cell death.Daptomycin is FDA-approved for treating complicated skin and soft tissue infections and bloodstream infections and right-sided infective endocarditis caused by S. aureus.
Serum triggers a high degree of tolerance to the lipopeptide antibiotic daptomycin and several other classes of antibiotic.Serum-induced daptomycin tolerance is due to two independent mechanisms. The first one is the activation of the GraRS two-component system. The activation is triggered by the host defense LL-37. So that, bacteria can make more peptidoglycan to make the cell wall become thicker. This can make the tolerance of bacteria. The second one is the increase of cardiolipin abundance in the membrane.The serum-adapted bacteria can change their membrane composition. This change can reduce the binding of daptomycin to the bacteria’s membrane.
Because of the high level of resistance to penicillins and because of the potential for MRSA to develop resistance to vancomycin, the U.S. Centers for Disease Control and Prevention has published guidelines for the appropriate use of vancomycin. In situations where the incidence of MRSA infections is known to be high, the attending physician may choose to use a glycopeptide antibiotic until the identity of the infecting organism is known. After the infection is confirmed to be due to a methicillin-susceptible strain of ''S. aureus'', treatment can be changed to flucloxacillin or even penicillin, as appropriate.
Vancomycin-resistant ''S. aureus'' (VRSA) is a strain of ''S. aureus'' that has become resistant to the glycopeptides. The first case of vancomycin-intermediate ''S. aureus'' (VISA) was reported in Japan in 1996; but the first case of ''S. aureus'' truly resistant to glycopeptide antibiotics was only reported in 2002. Three cases of VRSA infection had been reported in the United States as of 2005. At least in part the antimicrobial resistance in ''S. aureus'' can be explained by its ability to adapt. Multiple two component signal transduction pathways helps ''S. aureus'' to express genes that are required to survive under antimicrobial stress.
Efflux pumps
Among the various mechanisms that MRSA acquires to elude antibiotic resistance (e.g., drug inactivation, target alteration, reduction of permeability) there is also the overexpression of efflux pumps. Efflux pumps are membrane-integrated proteins that are physiologically needed in the cell for the exportation of xenobiotic compounds. They are divided into six families, each of which has a different structure, function, and transport of energy. The main efflux pumps of ''S. aureus'' are the MFS ( Major Facilitator Superfamily) which includes the MdeA pump as well as the NorA pump and the MATE (Multidrug and Toxin Extrusion) to which it belongs the MepA pump. For transport, these families use an electrochemical potential and an ion concentration gradient, while the ATP-binding cassette (ABC) family acquires its energy from the hydrolysis of ATP. These pumps are overexpressed by MDR ''S. aureus'' (Multidrug resistant ''S. aureus)'' and the result is an excessive expulsion of the antibiotic outside the cell, which makes its action ineffective. Efflux pumps also contribute significantly to the development of impenetrable biofilms. By directly modulating efflux pumps' activity or decreasing their expression, it may be possible to modify the resistant phenotype and restore the effectiveness of existing antibiotics.Carriage
About 33% of the U.S. population are carriers of ''S. aureus'' and about 2% carry MRSA. Even healthcare providers can be MRSA colonizers. The carriage of ''S. aureus'' is an important source of hospital-acquired infection (also called nosocomial) and community-acquired MRSA. Although ''S. aureus'' can be present on the skin of the host, a large proportion of its carriage is through the anterior nares of the nasal passages and can further be present in the ears. The ability of the nasal passages to harbour ''S. aureus'' results from a combination of a weakened or defective host immunity and the bacterium's ability to evade host innate immunity. Nasal carriage is also implicated in the occurrence of staph infections.Infection control
Environmental contamination is thought to play a relatively less important part compared to direct transmission. Emphasis on basichand washing
Hand washing (or handwashing), also known as hand hygiene, is the act of cleaning one's hands with soap, soap or handwash and water to remove viruses, bacteria, microorganisms, dirt, grease, and other harmful or unwanted substances stuck to th ...
techniques are, therefore, effective in preventing its transmission. The use of disposable aprons and gloves by staff reduces skin-to-skin contact, so further reduces the risk of transmission.
Recently, myriad cases of ''S. aureus'' have been reported in hospitals across America. Transmission of the pathogen is facilitated in medical settings where healthcare worker hygiene is insufficient. ''S. aureus'' is an incredibly hardy bacterium, as was shown in a study where it survived on polyester for just under three months; polyester is the main material used in hospital privacy curtains.
An important and previously unrecognized means of community-associated MRSA colonization and transmission is during sexual contact.
''Staphylococcus aureus'' is killed in one minute at 78 °C and in ten minutes at 64 °C but is resistant to freezing.
Certain strains of ''S. aureus'' have been described as being resistant to chlorine disinfection.
The use of mupirocin ointment can reduce the rate of infections due to nasal carriage of ''S. aureus.'' There is limited evidence that nasal decontamination of ''S. aureus'' using antibiotics or antiseptics can reduce the rates of surgical site infections.
Research
As of 2024, no approvedvaccine
A vaccine is a biological Dosage form, preparation that provides active acquired immunity to a particular infectious disease, infectious or cancer, malignant disease. The safety and effectiveness of vaccines has been widely studied and verifi ...
exists against ''S. aureus''. Early clinical trial
Clinical trials are prospective biomedical or behavioral research studies on human subject research, human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel v ...
s have been conducted for several vaccines candidates such as Nabi's StaphVax and PentaStaph, Intercell's / Merck's V710, VRi's SA75, and others.
While some of these vaccines candidates have shown immune responses, others aggravated an infection by ''S. aureus''. To date, none of these candidates provides protection against a ''S. aureus'' infection. The development of Nabi's StaphVax was stopped in 2005 after phase III trials failed. Intercell's first V710 vaccine variant was terminated during phase II/III after higher mortality and morbidity were observed among patients who developed ''S. aureus'' infection.
Nabi's enhanced ''S. aureus'' vaccines candidate PentaStaph was sold in 2011 to GlaxoSmithKline Biologicals S.A. The current status of PentaStaph is unclear. A WHO document indicates that PentaStaph failed in the phase III trial stage.
In 2010, GlaxoSmithKline started a phase 1 blind study to evaluate its GSK2392103A vaccine. As of 2016, this vaccine is no longer under active development.
Pfizer's ''S. aureus'' four-antigen vaccine SA4Ag was granted fast track designation by the U.S. Food and Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is respo ...
in February 2014. In 2015, Pfizer has commenced a phase 2b trial regarding the SA4Ag vaccine. Phase 1 results published in February 2017 showed a very robust and secure immunogenicity of SA4Ag. The vaccine underwent clinical trial until June 2019, with results published in September 2020, that did not demonstrate a significant reduction in Postoperative Bloodstream Infection after Surgery.
In 2015, Novartis Vaccines and Diagnostics, a former division of Novartis and now part of GlaxoSmithKline, published promising pre-clinical results of their four-component ''Staphylococcus aureus'' vaccine, 4C-staph.
In addition to vaccine development, research is being performed to develop alternative treatment options that are effective against antibiotic resistant strains including MRSA. Examples of alternative treatments are phage therapy, antimicrobial peptides
Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between Prokaryote, prokaryotic and eukaryota, eukaryotic cells that may ...
and host-directed therapy.
Standard strains
A number of standard strains of ''S. aureus'' (called "type cultures") are used in research and in laboratory testing, such as:See also
* Bundaberg tragedy, deaths of 12 children inoculated with an ''S. aureus''-contaminated vaccineReferences
Further reading
* *External links
StopMRSANow.org
— Discusses how to prevent the spread of MRSA
TheMRSA.com
— Understand what the MRSA infection is all about. * *
Type strain of ''Staphylococcus aureus'' at Bac''Dive'' – the Bacterial Diversity Metadatabase
{{Authority control Food microbiology aureus Bacteriology Gram-positive bacteria Bacterial diseases Pathogenic bacteria Healthcare-associated infections Bacteria described in 1884