Steroid and Lipid Hormones.svg on:
[Wikipedia]
[Google]
[Amazon]
A steroid is a biologically active organic compound with four rings arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and as

File:Testosteron.svg, alt=Chemical diagram, Testosterone, the principal male sex hormone and an
In addition to the ring scissions (cleavages), expansions and contractions (cleavage and reclosing to a larger or smaller rings)—all variations in the carbon-carbon bond framework—steroids can also vary:
* in the
 The hundreds of steroids found in animals, fungi, and plants are made from lanosterol (in animals and fungi; see examples above) or
The hundreds of steroids found in animals, fungi, and plants are made from lanosterol (in animals and fungi; see examples above) or
 The mevalonate pathway (also called HMG-CoA reductase pathway) begins with
The mevalonate pathway (also called HMG-CoA reductase pathway) begins with
 Steroidogenesis is the biological process by which steroids are generated from cholesterol and changed into other steroids. The pathways of steroidogenesis differ among species. The major classes of steroid hormones, as noted above (with their prominent members and functions), are the
Steroidogenesis is the biological process by which steroids are generated from cholesterol and changed into other steroids. The pathways of steroidogenesis differ among species. The major classes of steroid hormones, as noted above (with their prominent members and functions), are the
signaling molecules
In biology, cell signaling (cell signalling in British English) or cell communication is the ability of a cell to receive, process, and transmit signals with its environment and with itself. Cell signaling is a fundamental property of all cellula ...
. Hundreds of steroids are found in plants, animals and fungi. All steroids are manufactured in cells from the sterols
Sterol is an organic compound with formula , whose molecule is derived from that of gonane by replacement of a hydrogen atom in position 3 by a hydroxyl group. It is therefore an alcohol of gonane. More generally, any compounds that contain the gon ...
lanosterol ( opisthokonts) or cycloartenol
Cycloartenol is an important triterpenoid of the sterol class which is found in plants. It is the starting point for the synthesis of almost all plant steroids, making them chemically distinct from the steroids of fungi and animals, which are, ins ...
(plants). Lanosterol and cycloartenol are derived from the cyclization of the triterpene squalene.
The steroid core structure is typically composed of seventeen carbon atoms, bonded in four " fused" rings: three six-member cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colorless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
rings (rings A, B and C in the first illustration) and one five-member cyclopentane ring (the D ring). Steroids vary by the functional groups attached to this four-ring core and by the oxidation state of the rings. Sterol
Sterol is an organic compound with formula , whose molecule is derived from that of gonane by replacement of a hydrogen atom in position 3 by a hydroxyl group. It is therefore an alcohol of gonane. More generally, any compounds that contain the go ...
s are forms of steroids with a hydroxy group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
at position three and a skeleton derived from cholestane
Cholestane is a saturated tetracyclic triterpene. This 27-carbon biomarker is produced by diagenesis of cholesterol and is one of the most abundant biomarkers in the rock record. Presence of cholestane, its derivatives and related chemical compoun ...
. ''Also available with the same authors at'' ; ''Also available online at'' Steroids can also be more radically modified, such as by changes to the ring structure, for example, cutting one of the rings. Cutting Ring B produces secosteroids one of which is vitamin D3.
Examples include anabolic steroids
Anabolic steroids, also known more properly as anabolic–androgenic steroids (AAS), are steroidal androgens that include natural androgens like testosterone as well as synthetic androgens that are structurally related and have similar effects t ...
, the lipid cholesterol, the sex hormones estradiol and testosterone, and the anti-inflammatory drug dexamethasone.
Nomenclature


Gonane
Gonane is a chemical compound with formula , whose molecule can be described as three molecules or entities of cyclohexane and one of cyclopentane, fused in a particular way. More specifically, the molecule can be described as that of cyclohe ...
, also known as steran or cyclopentanoperhydrophenanthrene, the simplest steroid and the nucleus of all steroids and sterols, is composed of seventeen carbon atoms in carbon-carbon bonds forming four fused rings in a three-dimensional shape. The three cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colorless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
rings (A, B, and C in the first illustration) form the skeleton of a perhydro derivative of phenanthrene
Phenanthrene is a polycyclic aromatic hydrocarbon (PAH) with formula C14H10, consisting of three fused benzene rings. It is a colorless, crystal-like solid, but can also appear yellow. Phenanthrene is used to make dyes, plastics and pesticides, e ...
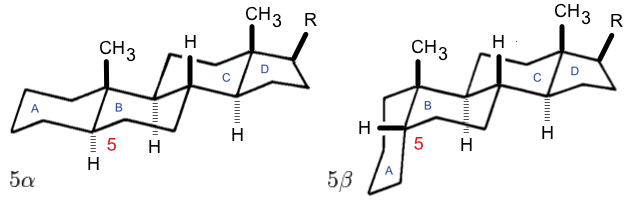
. The D ring has a cyclopentane structure. When the two methyl groups and eight carbon side chains (at C-17, as shown for cholesterol) are present, the steroid is said to have a cholestane framework. The two common 5α and 5β stereoisomeric forms of steroids exist because of differences in the side of the largely planar ring system where the hydrogen (H) atom at carbon-5 is attached, which results in a change in steroid A-ring conformation. Isomerisation at the C-21 side chain produces a parallel series of compounds, referred to as isosteroids.
Examples of steroid structures are:
anabolic steroid
Anabolic steroids, also known more properly as anabolic–androgenic steroids (AAS), are steroidal androgens that include natural androgens like testosterone (medication), testosterone as well as synthetic androgens that are structurally related ...
File:Cholsäure.svg, alt=Chemical diagram, Cholic acid, a bile acid
Bile acids are steroid acids found predominantly in the bile of mammals and other vertebrates. Diverse bile acids are synthesized in the liver. Bile acids are conjugated with taurine or glycine residues to give anions called bile salts.
Primary b ...
, showing the carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
and additional hydroxyl group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy g ...
s often present
File:Dexamethasone structure.svg, alt=Chemical diagram, Dexamethasone, a synthetic corticosteroid
Corticosteroids are a class of steroid hormones that are produced in the adrenal cortex of vertebrates, as well as the synthetic analogues of these hormones. Two main classes of corticosteroids, glucocorticoids and mineralocorticoids, are involv ...
drug
File:Lanosterin.svg, alt=Chemical diagram, Lanosterol, the biosynthetic precursor to animal steroids. The number of carbons (30) indicates its triterpenoid
Triterpenes are a class of chemical compounds composed of three terpene units with the molecular formula C30H48; they may also be thought of as consisting of six isoprene units. Animals, plants and fungi all produce triterpenes, including squale ...
classification.
File:Progesteron.svg, alt=Chemical diagram, Progesterone
Progesterone (P4) is an endogenous steroid and progestogen sex hormone involved in the menstrual cycle, pregnancy, and embryogenesis of humans and other species. It belongs to a group of steroid hormones called the progestogens and is the m ...
, a steroid hormone involved in the female menstrual cycle, pregnancy, and embryogenesis
File:Medrogestone.png, alt=Chemical diagram, Medrogestone, a synthetic drug with effects similar to progesterone
File:Sitosterol structure.svg, alt=Chemical diagram, β-Sitosterol, a plant or phytosterol, with a fully branched hydrocarbon side chain at C-17 and an hydroxyl group at C-3
bond order
In chemistry, bond order, as introduced by Linus Pauling, is defined as the difference between the number of bonds and anti-bonds.
The bond order itself is the number of electron pairs (covalent bonds) between two atoms. For example, in diat ...
s within the rings,
* in the number of methyl groups attached to the ring (and, when present, on the prominent side chain at C17),
* in the functional groups attached to the rings and side chain, and
* in the configuration
Configuration or configurations may refer to:
Computing
* Computer configuration or system configuration
* Configuration file, a software file used to configure the initial settings for a computer program
* Configurator, also known as choice board ...
of groups attached to the rings and chain.
For instance, sterol
Sterol is an organic compound with formula , whose molecule is derived from that of gonane by replacement of a hydrogen atom in position 3 by a hydroxyl group. It is therefore an alcohol of gonane. More generally, any compounds that contain the go ...
s such as cholesterol and lanosterol have a hydroxyl group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy g ...
attached at position C-3, while testosterone and progesterone
Progesterone (P4) is an endogenous steroid and progestogen sex hormone involved in the menstrual cycle, pregnancy, and embryogenesis of humans and other species. It belongs to a group of steroid hormones called the progestogens and is the m ...
have a carbonyl (oxo substituent) at C-3; of these, lanosterol alone has two methyl groups at C-4 and cholesterol (with a C-5 to C-6 double bond) differs from testosterone and progesterone (which have a C-4 to C-5 double bond).
Species distribution and function
Ineukaryote
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bacte ...
s, steroids are found in fungi, animals, and plants.
Fungal steroids
Fungal steroids include theergosterol
Ergosterol (ergosta-5,7,22-trien-3β-ol) is a sterol found in cell membranes of fungi and protozoa, serving many of the same functions that cholesterol serves in animal cells. Because many fungi and protozoa cannot survive without ergosterol, the ...
s, which are involved in maintaining the integrity of the fungal cellular membrane. Various antifungal drugs
An antifungal medication, also known as an antimycotic medication, is a pharmaceutical fungicide or fungistatic used to treat and prevent mycosis such as athlete's foot, ringworm, candidiasis (thrush), serious systemic infections such as Cryptoc ...
, such as amphotericin B and azole antifungals
Azoles are a class of five-membered heterocyclic compounds containing a nitrogen atom and at least one other non-carbon atom (i.e. nitrogen, sulfur, or oxygen) as part of the ring.
Their names originate from the Hantzsch–Widman nomenclature. Th ...
, utilize this information to kill pathogenic
In biology, a pathogen ( el, πάθος, "suffering", "passion" and , "producer of") in the oldest and broadest sense, is any organism or agent that can produce disease. A pathogen may also be referred to as an infectious agent, or simply a germ ...
fungi. Fungi can alter their ergosterol content (e.g. through loss of function mutations in the enzymes ERG3
ERG3 or sterol C-5 desaturase is a fungal enzyme originally from '' Saccharomyces cerevisiae'', the human ortholog of ERG3 is SC5D. ERG3 localizes to both the endoplasmic reticulum and vesicles, catalyzes the C5(6)-dehydrogenation of episterol ...
or ERG6, inducing depletion of ergosterol, or mutations that decrease the ergosterol content) to develop resistance to drugs that target ergosterol.
Ergosterol is analogous to the cholesterol found in the cellular membranes of animals (including humans), or the phytosterols found in the cellular membranes of plants. All mushrooms contain large quantities of ergosterol, in the range of tens to hundreds of milligrams per 100 grams of dry weight. Oxygen is necessary for the synthesis of ergosterol
Ergosterol (ergosta-5,7,22-trien-3β-ol) is a sterol found in cell membranes of fungi and protozoa, serving many of the same functions that cholesterol serves in animal cells. Because many fungi and protozoa cannot survive without ergosterol, the ...
in fungi.
Ergosterol is responsible for the vitamin D content found in mushrooms; ergosterol is chemically converted into provitamin D2 by exposure to ultraviolet light. Provitamin D2 spontaneously forms vitamin D2. However, not all fungi utilize ergosterol in their cellular membranes; for example, the pathogenic fungal species ''Pneumocystis jirovecii
''Pneumocystis jirovecii'' (previously ''P. carinii'') is a yeast-like fungus of the genus ''Pneumocystis''. The causative organism of ''Pneumocystis'' pneumonia, it is an important human pathogen, particularly among immunocompromised hosts. Pr ...
'' does not, which has important clinical implications (given the mechanism of action of many antifungal drugs). Using the fungus '' Saccharomyces cerevisiae'' as an example, other major steroids include ergosta‐5,7,22,24(28)‐tetraen‐3β‐ol, zymosterol
Zymosterol is an intermediate in cholesterol biosynthesis. Disregarding some intermediate compounds (e.g. 4-4-dimethylzymosterol) lanosterol can be considered a precursor of zymosterol in the cholesterol synthesis pathway. The conversion of zymost ...
, and lanosterol. ''S. cerevisiae'' utilizes 5,6‐dihydroergosterol in place of ergosterol in its cell membrane.
Animal steroids
Animal steroids include compounds of vertebrate and insect origin, the latter including ecdysteroids such as ecdysterone (controlling molting in some species). Vertebrate examples include the steroid hormones and cholesterol; the latter is a structural component ofcell membranes
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment ( ...
that helps determine the fluidity of cell membranes
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment ( ...
and is a principal constituent of plaque (implicated in atherosclerosis). Steroid hormones include:
* Sex hormones, which influence sex differences
Sexual dimorphism is the condition where the sexes of the same animal and/or plant species exhibit different morphological characteristics, particularly characteristics not directly involved in reproduction. The condition occurs in most ani ...
and support reproduction
Reproduction (or procreation or breeding) is the biological process by which new individual organisms – "offspring" – are produced from their "parent" or parents. Reproduction is a fundamental feature of all known life; each individual or ...
. These include androgens, estrogens, and progestogen
Progestogens, also sometimes written progestagens or gestagens, are a class of natural or synthetic steroid hormones that bind to and activate the progesterone receptors (PR). Progesterone is the major and most important progestogen in the body. ...
s.
* Corticosteroid
Corticosteroids are a class of steroid hormones that are produced in the adrenal cortex of vertebrates, as well as the synthetic analogues of these hormones. Two main classes of corticosteroids, glucocorticoids and mineralocorticoids, are involv ...
s, including most synthetic steroid drugs, with natural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical syn ...
classes the glucocorticoids (which regulate many aspects of metabolism and immune function
The immune system is a network of biological processes that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to parasitic worms, as well as cancer cells and objects such as wood splinte ...
) and the mineralocorticoid
Mineralocorticoids are a class of corticosteroids, which in turn are a class of steroid hormones. Mineralocorticoids are produced in the adrenal cortex and influence salt and water balances (electrolyte balance and fluid balance). The primary mi ...
s (which help maintain blood volume and control renal excretion of electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dis ...
s)
* Anabolic steroid
Anabolic steroids, also known more properly as anabolic–androgenic steroids (AAS), are steroidal androgens that include natural androgens like testosterone (medication), testosterone as well as synthetic androgens that are structurally related ...
s, natural and synthetic, which interact with androgen receptors to increase muscle and bone synthesis. In popular use, the term "steroids" often refers to anabolic steroids.
Plant steroids
Plant steroids include steroidal alkaloids found inSolanaceae
The Solanaceae , or nightshades, are a family of flowering plants that ranges from annual and perennial herbs to vines, lianas, epiphytes, shrubs, and trees, and includes a number of agricultural crops, medicinal plants, spices, weeds, and orn ...
and Melanthiaceae (specially the genus Veratrum), cardiac glycosides, the phytosterols and the brassinosteroids (which include several plant hormones).
Prokaryotes
Inprokaryotes
A prokaryote () is a single-celled organism that lacks a nucleus and other membrane-bound organelles. The word ''prokaryote'' comes from the Greek πρό (, 'before') and κάρυον (, 'nut' or 'kernel').Campbell, N. "Biology:Concepts & Connec ...
, biosynthetic pathways exist for the tetracyclic steroid framework (e.g. in mycobacteria
''Mycobacterium'' is a genus of over 190 species in the phylum Actinomycetota, assigned its own family, Mycobacteriaceae. This genus includes pathogens known to cause serious diseases in mammals, including tuberculosis ('' M. tuberculosis'') and ...
) – where its origin from eukaryote
Eukaryotes () are organisms whose cells have a nucleus. All animals, plants, fungi, and many unicellular organisms, are Eukaryotes. They belong to the group of organisms Eukaryota or Eukarya, which is one of the three domains of life. Bacte ...
s is conjectured – and the more-common pentacyclic triterpinoid hopanoid
Hopanoids are a diverse subclass of triterpenoids with the same hydrocarbon skeleton as the compound hopane. This group of pentacyclic molecules therefore refers to simple hopenes, hopanols and hopanes, but also to extensively functionalized deriva ...
framework.
Types
By function
The major classes of steroid hormones, with prominent members and examples of related functions, are: *Corticosteroid
Corticosteroids are a class of steroid hormones that are produced in the adrenal cortex of vertebrates, as well as the synthetic analogues of these hormones. Two main classes of corticosteroids, glucocorticoids and mineralocorticoids, are involv ...
s:
** Glucocorticoids:
*** Cortisol
Cortisol is a steroid hormone, in the glucocorticoid class of hormones. When used as a medication, it is known as hydrocortisone.
It is produced in many animals, mainly by the ''zona fasciculata'' of the adrenal cortex in the adrenal gland ...
, a glucocorticoid whose functions include immunosuppression
** Mineralocorticoid
Mineralocorticoids are a class of corticosteroids, which in turn are a class of steroid hormones. Mineralocorticoids are produced in the adrenal cortex and influence salt and water balances (electrolyte balance and fluid balance). The primary mi ...
s:
*** Aldosterone
Aldosterone is the main mineralocorticoid steroid hormone produced by the zona glomerulosa of the adrenal cortex in the adrenal gland. It is essential for sodium conservation in the kidney, salivary glands, sweat glands, and colon. It plays a c ...
, a mineralocorticoid
Mineralocorticoids are a class of corticosteroids, which in turn are a class of steroid hormones. Mineralocorticoids are produced in the adrenal cortex and influence salt and water balances (electrolyte balance and fluid balance). The primary mi ...
that helps regulate blood pressure
Blood pressure (BP) is the pressure of circulating blood against the walls of blood vessels. Most of this pressure results from the heart pumping blood through the circulatory system. When used without qualification, the term "blood pressure" r ...
through water and electrolyte balance
* Sex steroid
Sex hormones, also known as sex steroids, gonadocorticoids and gonadal steroids, are steroid hormones that interact with vertebrate steroid hormone receptors. The sex hormones include the androgens, estrogens, and progestogens. Their effects are ...
s:
** Progestogen
Progestogens, also sometimes written progestagens or gestagens, are a class of natural or synthetic steroid hormones that bind to and activate the progesterone receptors (PR). Progesterone is the major and most important progestogen in the body. ...
s:
*** Progesterone
Progesterone (P4) is an endogenous steroid and progestogen sex hormone involved in the menstrual cycle, pregnancy, and embryogenesis of humans and other species. It belongs to a group of steroid hormones called the progestogens and is the m ...
, which regulates cyclical changes in the endometrium
The endometrium is the inner epithelial layer, along with its mucous membrane, of the mammalian uterus. It has a basal layer and a functional layer: the basal layer contains stem cells which regenerate the functional layer. The functional laye ...
of the uterus and maintains a pregnancy
** Androgens:
*** Testosterone, which contributes to the development and maintenance of male secondary sex characteristics
** Estrogens:
*** Estradiol, which contributes to the development and maintenance of female secondary sex characteristics
Additional classes of steroids include:
* Neurosteroid
Neurosteroids, also known as neuroactive steroids, are endogenous or exogenous steroids that rapidly alter neuronal excitability through interaction with ligand-gated ion channels and other cell surface receptors. The term ''neurosteroid'' was coin ...
s such as and allopregnanolone
Allopregnanolone is a naturally occurring neurosteroid which is made in the body from the hormone progesterone. As a medication, allopregnanolone is referred to as brexanolone, sold under the brand name Zulresso, and used to treat postpartum d ...
* Bile acid
Bile acids are steroid acids found predominantly in the bile of mammals and other vertebrates. Diverse bile acids are synthesized in the liver. Bile acids are conjugated with taurine or glycine residues to give anions called bile salts.
Primary b ...
s such as taurocholic acid
* Aminosteroid neuromuscular blocking agents (mainly synthetic) such as pancuronium bromide
* Steroidal antiandrogen
A steroidal antiandrogen (SAA) is an antiandrogen with a steroidal chemical structure. They are typically antagonists of the androgen receptor (AR) and act both by blocking the effects of androgens like testosterone and dihydrotestosterone (DHT) ...
s (mainly synthetic) such as cyproterone acetate
* Steroidogenesis inhibitors (mainly exogenous) such as alfatradiol
Alfatradiol, also known as 17α-estradiol and sold under the brand names Avicis, Avixis, Ell-Cranell Alpha, and Pantostin, is a weak estrogen and 5α-reductase inhibitor medication which is used topically in the treatment of pattern hair loss ( ...
* Membrane sterols such as cholesterol, ergosterol
Ergosterol (ergosta-5,7,22-trien-3β-ol) is a sterol found in cell membranes of fungi and protozoa, serving many of the same functions that cholesterol serves in animal cells. Because many fungi and protozoa cannot survive without ergosterol, the ...
, and various phytosterols
* Toxins such as steroidal saponin
Saponins (Latin "sapon", soap + "-in", one of), also selectively referred to as triterpene glycosides, are bitter-tasting usually toxic plant-derived organic chemicals that have a foamy quality when agitated in water. They are widely distributed ...
s and cardenolides/ cardiac glycosides
As well as the following class of secosteroids (open-ring steroids):
* Vitamin D forms such as ergocalciferol, cholecalciferol, and calcitriol
By structure
Intact ring system
Steroids can be classified based on their chemical composition. One example of how MeSH performs this classification is available at the Wikipedia MeSH catalog. Examples of this classification include: In biology, it is common to name the above steroid classes by the number of carbon atoms present when referring to hormones: C18-steroids for the estranes (mostly estrogens), C19-steroids for the androstanes (mostly androgens), and C21-steroids for the pregnanes (mostly corticosteroids). The classification "17-ketosteroid
150px, Androstenedione
150px, Androsterone
150px, Estrone
A ketosteroid, or an oxosteroid, is a steroid in which a hydrogen atom has been replaced with a ketone (C=O) group.
A 17-ketosteroid is a ketosteroid in which the ketone is located spe ...
" is also important in medicine.
The gonane (steroid nucleus) is the parent 17-carbon tetracyclic hydrocarbon molecule with no alkyl sidechains.
Cleaved, contracted, and expanded rings
Secosteroids (Latin ''seco'', "to cut") are a subclass of steroidal compounds resulting, biosynthetically or conceptually, from scission (cleavage) of parent steroid rings (generally one of the four). Major secosteroid subclasses are defined by the steroid carbon atoms where this scission has taken place. For instance, the prototypical secosteroid cholecalciferol, vitamin D3 (shown), is in the 9,10-secosteroid subclass and derives from the cleavage of carbon atoms C-9 and C-10 of the steroid B-ring; 5,6-secosteroids and 13,14-steroids are similar.Norsteroid
''Norsteroids'' (nor-, L. ''norma'', from "normal" in chemistry, indicating carbon removal) are a structural class of steroids that have had an atom or atoms (typically carbon) removed, biosynthetically or synthetically, from positions of branch ...
s ( nor-, L. ''norma''; "normal" in chemistry, indicating carbon removal) and homosteroids (homo-, Greek ''homos''; "same", indicating carbon addition) are structural subclasses of steroids formed from biosynthetic steps. The former involves enzymic ring expansion-contraction reactions, and the latter is accomplished ( biomimetically) or (more frequently) through ring closure
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where al ...
s of acyclic precursors with more (or fewer) ring atoms than the parent steroid framework.
Combinations of these ring alterations are known in nature. For instance, ewes who graze on corn lily ingest cyclopamine (shown) and veratramine
Veratramine is an alkaloid
Alkaloids are a class of basic, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some sy ...
, two of a sub-family of steroids where the C- and D-rings are contracted and expanded respectively via a biosynthetic migration of the original C-13 atom. Ingestion of these C-nor-D-homosteroids results in birth defects in lambs: cyclopia from cyclopamine and leg deformity from veratramine. A further C-nor-D-homosteroid (nakiterpiosin) is excreted by Okinawan cyanobacteriosponges. e.g., ''Terpios
''Terpios'' is a genus of sea sponges belonging to the family Suberitidae.
Species
*'' Terpios aploos'' de Laubenfels, 1954
*'' Terpios australiensis'' Hentschel, 1909
*'' Terpios belindae'' Rützler & Smith, 1993
*'' Terpios cruciata'' (Dendy, ...
hoshinota'', leading to coral mortality from black coral disease. Nakiterpiosin-type steroids are active against the signaling pathway involving the smoothened and hedgehog proteins, a pathway which is hyperactive in a number of cancers.
Biological significance
Steroids and their metabolites often function as signalling molecules (the most notable examples are steroid hormones), and steroids andphospholipid
Phospholipids, are a class of lipids whose molecule has a hydrophilic "head" containing a phosphate group and two hydrophobic "tails" derived from fatty acids, joined by an alcohol residue (usually a glycerol molecule). Marine phospholipids typ ...
s are components of cell membranes. Steroids such as cholesterol decrease membrane fluidity.
Similar to lipids, steroids are highly concentrated energy stores. However, they are not typically sources of energy; in mammals, they are normally metabolized and excreted.
Steroids play critical roles in a number of disorders, including malignancies like prostate cancer
Prostate cancer is cancer of the prostate. Prostate cancer is the second most common cancerous tumor worldwide and is the fifth leading cause of cancer-related mortality among men. The prostate is a gland in the male reproductive system that sur ...
, where steroid production inside and outside the tumour promotes cancer cell aggressiveness.
Biosynthesis and metabolism
cycloartenol
Cycloartenol is an important triterpenoid of the sterol class which is found in plants. It is the starting point for the synthesis of almost all plant steroids, making them chemically distinct from the steroids of fungi and animals, which are, ins ...
(in other eukaryotes). Both lanosterol and cycloartenol derive from cyclization of the triterpenoid
Triterpenes are a class of chemical compounds composed of three terpene units with the molecular formula C30H48; they may also be thought of as consisting of six isoprene units. Animals, plants and fungi all produce triterpenes, including squale ...
squalene. Lanosterol and cycloartenol are sometimes called protosterols because they serve as the starting compounds for all other steroids.
Steroid biosynthesis is an anabolic pathway which produces steroids from simple precursors. A unique biosynthetic pathway is followed in animals (compared to many other organisms), making the pathway a common target for antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of ...
s and other anti-infection drugs. Steroid metabolism in humans is also the target of cholesterol-lowering drugs, such as statins. In humans and other animals the biosynthesis of steroids follows the mevalonate pathway, which uses acetyl-CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized for ...
as building blocks for dimethylallyl diphosphate
Dimethylallyl pyrophosphate (DMAPP; or alternatively, dimethylallyl diphosphate (DMADP); also isoprenyl pyrophosphate) is an isoprenoid precursor. It is a product of both the mevalonate pathway and the MEP pathway of isoprenoid precursor biosynt ...
(DMAPP) and isopentenyl diphosphate
Isopentenyl pyrophosphate (IPP, isopentenyl diphosphate, or IDP) is an isoprenoid precursor. IPP is an intermediate in the classical, HMG-CoA reductase pathway (commonly called the mevalonate pathway) and in the ''non-mevalonate'' MEP pathway of ...
(IPP).
In subsequent steps DMAPP and IPP conjugate to form farnesyl diphosphate
Farnesyl pyrophosphate (FPP), also known as farnesyl diphosphate (FDP), is an intermediate in the biosynthesis of terpenes and terpenoids such as sterols and carotenoids. It is also used in the synthesis of CoQ (part of the electron transport cha ...
(FPP), which further conjugates with each other to form the linear triterpenoid squalene. Squalene biosynthesis is catalyzed by squalene synthase
Squalene synthase (SQS) or farnesyl-diphosphate:farnesyl-diphosphate farnesyl transferase is an enzyme localized to the membrane of the endoplasmic reticulum. SQS participates in the isoprenoid biosynthetic pathway, catalyzing a two-step react ...
, which belongs to the squalene/phytoene synthase family
The squalene/phytoene synthase family represents proteins that catalyze the head-to-head condensation of C15 and C20 prenyl units (i.e. farnesyl diphosphate and genranylgeranyl diphosphate). This enzymatic step constitutes part of steroid and c ...
. Subsequent epoxidation and cyclization of squalene generate lanosterol, which is the starting point for additional modifications into other steroids (steroidogenesis). In other eukaryotes, the cyclization product of epoxidized squalene (oxidosqualene) is cycloartenol.
Mevalonate pathway
acetyl-CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized for ...
and ends with dimethylallyl diphosphate
Dimethylallyl pyrophosphate (DMAPP; or alternatively, dimethylallyl diphosphate (DMADP); also isoprenyl pyrophosphate) is an isoprenoid precursor. It is a product of both the mevalonate pathway and the MEP pathway of isoprenoid precursor biosynt ...
(DMAPP) and isopentenyl diphosphate
Isopentenyl pyrophosphate (IPP, isopentenyl diphosphate, or IDP) is an isoprenoid precursor. IPP is an intermediate in the classical, HMG-CoA reductase pathway (commonly called the mevalonate pathway) and in the ''non-mevalonate'' MEP pathway of ...
(IPP).
DMAPP and IPP donate isoprene
Isoprene, or 2-methyl-1,3-butadiene, is a common volatile organic compound with the formula CH2=C(CH3)−CH=CH2. In its pure form it is a colorless volatile liquid. Isoprene is an unsaturated hydrocarbon. It is produced by many plants and animals ...
units, which are assembled and modified to form terpenes and isoprenoids
The terpenoids, also known as isoprenoids, are a class of naturally occurring organic chemicals derived from the 5-carbon compound isoprene and its derivatives called terpenes, diterpenes, etc. While sometimes used interchangeably with "terpenes", ...
(a large class of lipids, which include the carotenoid
Carotenoids (), also called tetraterpenoids, are yellow, orange, and red organic compound, organic pigments that are produced by plants and algae, as well as several bacteria, and Fungus, fungi. Carotenoids give the characteristic color to pumpki ...
s and form the largest class of plant natural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical syn ...
s. Here, the isoprene units are joined to make squalene and folded into a set of rings to make lanosterol. Lanosterol can then be converted into other steroids, such as cholesterol and ergosterol
Ergosterol (ergosta-5,7,22-trien-3β-ol) is a sterol found in cell membranes of fungi and protozoa, serving many of the same functions that cholesterol serves in animal cells. Because many fungi and protozoa cannot survive without ergosterol, the ...
.
Two classes of drugs
A drug is any chemical substance that causes a change in an organism's physiology or psychology when consumed. Drugs are typically distinguished from food and substances that provide nutritional support. Consumption of drugs can be via inhalat ...
target the mevalonate pathway: statins (like rosuvastatin
Rosuvastatin, sold under the brand name Crestor among others, is a statin medication, used to prevent cardiovascular disease in those at high risk and treat abnormal lipids. It is recommended to be used together with dietary changes, exercise, ...
), which are used to reduce elevated cholesterol levels, and bisphosphonates (like zoledronate), which are used to treat a number of bone-degenerative diseases.
Steroidogenesis
progestogen
Progestogens, also sometimes written progestagens or gestagens, are a class of natural or synthetic steroid hormones that bind to and activate the progesterone receptors (PR). Progesterone is the major and most important progestogen in the body. ...
s, corticosteroid
Corticosteroids are a class of steroid hormones that are produced in the adrenal cortex of vertebrates, as well as the synthetic analogues of these hormones. Two main classes of corticosteroids, glucocorticoids and mineralocorticoids, are involv ...
s (corticoids), androgens, and estrogens. Human steroidogenesis of these classes occurs in a number of locations:
*Progestogens are the precursors of all other human steroids, and all human tissues which produce steroids must first convert cholesterol to pregnenolone. This conversion is the rate-limiting step of steroid synthesis, which occurs inside the mitochondrion
A mitochondrion (; ) is an organelle found in the cells of most Eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used ...
of the respective tissue.
*Cortisol, corticosterone, aldosterone, and testosterone are produced in the adrenal cortex
The adrenal cortex is the outer region and also the largest part of an adrenal gland. It is divided into three separate zones: zona glomerulosa, zona fasciculata and zona reticularis. Each zone is responsible for producing specific hormones. It is ...
.
*Estradiol, estrone and progesterone are made primarily in the ovary
The ovary is an organ in the female reproductive system that produces an ovum. When released, this travels down the fallopian tube into the uterus, where it may become fertilized by a sperm. There is an ovary () found on each side of the body. ...
, estriol in placenta during pregnancy, and testosterone primarily in the testes (some testosterone is also produced in the adrenal cortex).
*Estradiol is converted from testosterone directly (in males), or via the primary pathway DHEA - androstenedione - estrone and secondarily via testosterone (in females).
*Stromal cells
Stromal cells, or mesenchymal stromal cells, are differentiating cells found in abundance within bone marrow but can also be seen all around the body. Stromal cells can become connective tissue cells of any organ, for example in the uterine mucos ...
have been shown to produce steroids in response to signaling produced by androgen-starved prostate cancer
Prostate cancer is cancer of the prostate. Prostate cancer is the second most common cancerous tumor worldwide and is the fifth leading cause of cancer-related mortality among men. The prostate is a gland in the male reproductive system that sur ...
cells.
*Some neurons
A neuron, neurone, or nerve cell is an electrically excitable cell that communicates with other cells via specialized connections called synapses. The neuron is the main component of nervous tissue in all animals except sponges and placozoa. N ...
and glia in the central nervous system (CNS) express the enzymes
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecule ...
required for the local synthesis of pregnenolone, progesterone, DHEA and DHEAS, ''de novo'' or from peripheral sources.
Alternative pathways
In plants and bacteria, thenon-mevalonate pathway
The non-mevalonate pathway—also appearing as the mevalonate-independent pathway and the 2-''C''-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate (MEP/DOXP) pathway—is an alternative metabolic pathway for the biosynthesis of the is ...
(MEP pathway) uses pyruvate
Pyruvic acid (CH3COCOOH) is the simplest of the alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate base, CH3COCOO−, is an intermediate in several metabolic pathways throughout the cell.
Pyruvic aci ...
and glyceraldehyde 3-phosphate as substrates to produce IPP and DMAPP.
During diseases pathways otherwise not significant in healthy humans can become utilized. For example, in one form of congenital adrenal hyperplasia a deficiency in the 21-hydroxylase enzymatic pathway leads to an excess of 17α-Hydroxyprogesterone
17α-Hydroxyprogesterone (17α-OHP), also known as 17-OH progesterone (17-OHP), or hydroxyprogesterone (OHP), is an endogenous progestogen steroid hormone related to progesterone. It is also a chemical intermediate in the biosynthesis of many ot ...
(17-OHP) – this pathological excess of 17-OHP in turn may be converted to dihydrotestosterone (DHT, a potent androgen) through among others 17,20 Lyase
Cytochrome P450 17A1 (steroid 17α-monooxygenase, 17α-hydroxylase, 17-alpha-hydroxylase, 17,20-lyase, 17,20-desmolase) is an enzyme of the hydroxylase type that in humans is encoded by the ''CYP17A1'' gene on chromosome 10. It is ubiquitously expr ...
(a member of the cytochrome P450
Cytochromes P450 (CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are ...
family of enzymes), 5α-Reductase
5α-Reductases, also known as 3-oxo-5α-steroid 4-dehydrogenases, are enzymes involved in steroid metabolism. They participate in three metabolic pathways: bile acid biosynthesis, androgen and estrogen metabolism. There are three isozymes of ...
and 3α-Hydroxysteroid dehydrogenase.
Catabolism and excretion
Steroids are primarily oxidized by cytochrome P450 oxidase enzymes, such asCYP3A4
Cytochrome P450 3A4 (abbreviated CYP3A4) () is an important enzyme in the body, mainly found in the liver and in the intestine. It oxidizes small foreign organic molecules (xenobiotics), such as toxins or drugs, so that they can be removed from t ...
. These reactions introduce oxygen into the steroid ring, allowing the cholesterol to be broken up by other enzymes into bile acids. These acids can then be eliminated by secretion from the liver in bile
Bile (from Latin ''bilis''), or gall, is a dark-green-to-yellowish-brown fluid produced by the liver of most vertebrates that aids the digestion of lipids in the small intestine. In humans, bile is produced continuously by the liver (liver bile ...
. The expression of the oxidase
In biochemistry, an oxidase is an enzyme that catalyzes oxidation-reduction reactions, especially one involving dioxygen (O2) as the electron acceptor. In reactions involving donation of a hydrogen atom, oxygen is reduced to water (H2O) or hydro ...
gene can be upregulated by the steroid sensor PXR when there is a high blood concentration of steroids. Steroid hormones, lacking the side chain of cholesterol and bile acids, are typically hydroxylated
In chemistry, hydroxylation can refer to:
*(i) most commonly, hydroxylation describes a chemistry, chemical process that introduces a hydroxyl group () into an organic compound.
*(ii) the ''degree of hydroxylation'' refers to the number of OH gr ...
at various ring positions or oxidized at the 17 position, conjugated with sulfate or glucuronic acid
Glucuronic acid (from Greek γλεῦκος "''wine, must''" and οὖρον "''urine''") is a uronic acid that was first isolated from urine (hence the name). It is found in many gums such as gum arabic (c. 18%), xanthan, and kombucha tea and ...
and excreted in the urine.
Isolation, structure determination, and methods of analysis
Steroid ''isolation'', depending on context, is the isolation of chemical matter required for chemical structure elucidation, derivitzation or degradation chemistry, biological testing, and other research needs (generally milligrams to grams, but often more or the isolation of "analytical quantities" of the substance of interest (where the focus is on identifying and quantifying the substance (for example, in biological tissue or fluid). The amount isolated depends on the analytical method, but is generally less than one microgram. The methods of isolation to achieve the two scales of product are distinct, but includeextraction Extraction may refer to:
Science and technology
Biology and medicine
* Comedo extraction, a method of acne treatment
* Dental extraction, the surgical removal of a tooth from the mouth
Computing and information science
* Data extraction, the pro ...
, precipitation, adsorption, chromatography, and crystallization
Crystallization is the process by which solid forms, where the atoms or molecules are highly organized into a structure known as a crystal. Some ways by which crystals form are precipitating from a solution, freezing, or more rarely deposi ...
. In both cases, the isolated substance is purified to chemical homogeneity; combined separation and analytical methods, such as LC-MS, are chosen to be "orthogonal"—achieving their separations based on distinct modes of interaction between substance and isolating matrix—to detect a single species in the pure sample.
''Structure determination'' refers to the methods to determine the chemical structure of an isolated pure steroid, using an evolving array of chemical and physical methods which have included NMR and small-molecule crystallography
Crystallography is the experimental science of determining the arrangement of atoms in crystalline solids. Crystallography is a fundamental subject in the fields of materials science and solid-state physics (condensed matter physics). The wor ...
. ''Methods of analysis'' overlap both of the above areas, emphasizing analytical methods to determining if a steroid is present in a mixture and determining its quantity.
Chemical synthesis
Microbialcatabolism
Catabolism () is the set of metabolic pathways that breaks down molecules into smaller units that are either oxidized to release energy or used in other anabolic reactions. Catabolism breaks down large molecules (such as polysaccharides, lipids, ...
of phytosterol side chains yields C-19 steroids, C-22 steroids, and 17-ketosteroid
150px, Androstenedione
150px, Androsterone
150px, Estrone
A ketosteroid, or an oxosteroid, is a steroid in which a hydrogen atom has been replaced with a ketone (C=O) group.
A 17-ketosteroid is a ketosteroid in which the ketone is located spe ...
s (i.e. precursors to adrenocortical hormone In humans and other animals, the adrenocortical hormones are hormones produced by the adrenal cortex, the outer region of the adrenal gland. These polycyclic steroid hormones have a variety of roles that are crucial for the body’s response to stre ...
s and contraceptive
Birth control, also known as contraception, anticonception, and fertility control, is the use of methods or devices to prevent unwanted pregnancy. Birth control has been used since ancient times, but effective and safe methods of birth contr ...
s). The addition and modification of functional groups is key when producing the wide variety of medications available within this chemical classification. These modifications are performed using conventional organic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
and/or biotransformation techniques.
Precursors
Semisynthesis
Thesemisynthesis
Semisynthesis, or partial chemical synthesis, is a type of chemical synthesis that uses chemical compounds isolated from natural sources (such as microbial cell cultures or plant material) as the starting materials to produce novel compounds with ...
of steroids often begins from precursors such as cholesterol, phytosterols, or sapogenin
Sapogenins are the aglycones, or non-saccharide, portions of the family of natural products known as saponins. Sapogenins contain steroid or other triterpene frameworks as their key organic feature. For example, steroidal sapogenins such as tigge ...
s. The efforts of Syntex Laboratorios Syntex SA (later Syntex Laboratories, Inc.) was a pharmaceutical company formed in Mexico City in January 1944 by Russell Marker, Emeric Somlo, and Federico Lehmann to manufacture therapeutic steroids from the Mexican yams called ''cabe ...
, a company involved in the Mexican barbasco trade, used '' Dioscorea mexicana'' to produce the sapogenin diosgenin
Diosgenin, a phytosteroid sapogenin, is the product of hydrolysis by acids, strong bases, or enzymes of saponins, extracted from the tubers of ''Dioscorea'' wild yam species, such as the Kokoro. The sugar-free (aglycone) product of such hydrolys ...
in the early days of the synthetic steroid pharmaceutical industry.
Total synthesis
Some steroidal hormones are economically obtained only by total synthesis from petrochemicals (e.g. 13- alkyl steroids). For example, the pharmaceuticalNorgestrel
Norgestrel, sold under the brand name Ovral among others, is a progestin medication which is used in birth control pills and in menopausal hormone therapy. It is available both in combination with an estrogen and alone. It is taken by mouth.
S ...
begins from methoxy-1-tetralone
1-Tetralone is a bicyclic aromatic hydrocarbon and a ketone. In terms of its structure, it can also be regarded as benzo-fused cyclohexanone. It is a colorless oil with a faint odor. It is used as starting material for agricultural and pharmace ...
, a petrochemical derived from phenol.
Research awards
A number of Nobel Prizes have been awarded for steroid research, including: * 1927 (Chemistry
Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ...
) Heinrich Otto Wieland
Heinrich Otto Wieland (; 4 June 1877 – 5 August 1957) was a German chemist. He won the 1927 Nobel Prize in Chemistry for his research into the bile acids.
Career
In 1901 Wieland received his doctorate at the University of Munich while studyi ...
— Constitution of bile acids and sterols and their connection to vitamins
* 1928 (Chemistry) Adolf Otto Reinhold Windaus
Adolf Otto Reinhold Windaus (; 25 December 1876 – 9 June 1959) was a German chemist who won a Nobel Prize in Chemistry in 1928 for his work on sterols and their relation to vitamins. He was the doctoral advisor of Adolf Butenandt who also won ...
— Constitution of sterols and their connection to vitamins
* 1939 (Chemistry) Adolf Butenandt and Leopold Ružička — Isolation and structural studies of steroid sex hormones, and related studies on higher terpenes
* 1950 ( Physiology or Medicine) Edward Calvin Kendall, Tadeus Reichstein, and Philip Hench
Philip Showalter Hench (February 28, 1896 – March 30, 1965) was an American physician. Hench, along with his Mayo Clinic co-worker Edward Calvin Kendall and Swiss chemist Tadeus Reichstein was awarded the Nobel Prize for Physiology or Medicine ...
— Structure and biological effects of adrenal hormones
* 1965 (Chemistry) Robert Burns Woodward — In part, for the synthesis of cholesterol, cortisone, and lanosterol
* 1969 (Chemistry) Derek Barton and Odd Hassel
Odd Hassel (17 May 1897 – 11 May 1981) was a Norwegian physical chemist and Nobel Laureate.
Biography
Hassel was born in Kristiania (now Oslo), Norway. His parents were Ernst Hassel (1848–1905), a gynaecologist, and Mathilde Klaveness ( ...
— Development of the concept of conformation in chemistry, emphasizing the steroid nucleus
* 1975 (Chemistry) Vladimir Prelog — In part, for developing methods to determine the stereochemical course of cholesterol biosynthesis from mevalonic acid
Mevalonic acid (MVA) is a key organic compound in biochemistry; the name is a contraction of dihydroxymethylvalerolactone. The carboxylate anion of mevalonic acid, which is the predominant form in biological environments, is known as ''mevalonate ...
via squalene
See also
* Adrenal gland * Batrachotoxin *List of steroid abbreviations
The steroid hormone
A hormone (from the Greek participle , "setting in motion") is a class of signaling molecules in multicellular organisms that are sent to distant organs by complex biological processes to regulate physiology and behavior. ...
* List of steroids
List of steroids may refer to:
* List of androgens/anabolic steroids – steroidal androgens/anabolic steroids
* List of androgens/anabolic steroids (alternate) – steroidal androgens/anabolic steroids
* List of steroidal antiandrogens – st ...
* Membrane steroid receptor Membrane steroid receptors (mSRs), also called extranuclear steroid receptors, are a class of cell surface receptors activated by endogenous steroids that mediate rapid, non-genomic signaling via modulation of intracellular signaling cascades. mSR ...
* Pheromone
* Reverse cholesterol transport
Reverse cholesterol transport is a multi-step process resulting in the net movement of cholesterol from peripheral tissues back to the liver first via entering the lymphatic system, then the bloodstream.
Cholesterol from non-hepatic peripheral ti ...
* Steroidogenesis inhibitor
* Steroidogenic acute regulatory protein
The steroidogenic acute regulatory protein, commonly referred to as StAR (STARD1), is a transport protein that regulates cholesterol transfer within the mitochondria, which is the rate-limiting step in the production of steroid hormones. It is p ...
* Steroidogenic enzyme
References
Bibliography
* * * A concise history of the study of steroids. * A review of the history of steroid synthesis, especially biomimetic. * Adrenal steroidogenesis pathway. * * {{Authority control Metabolic pathways Wikipedia articles with sections published in WikiJournal of Medicine