Boltzmann2.jpg on:
[Wikipedia]
[Google]
[Amazon]
Ludwig Eduard Boltzmann (; 20 February 1844 – 5 September 1906) was an Austrian physicist and
 In 1872, long before women were admitted to Austrian universities, he met Henriette von Aigentler, an aspiring teacher of mathematics and physics in Graz. She was refused permission to audit lectures unofficially. Boltzmann supported her decision to appeal, which was successful. On 17 July 1876 Ludwig Boltzmann married Henriette; they had three daughters: Henriette (1880), Ida (1884) and Else (1891); and a son, Arthur Ludwig (1881). Boltzmann went back to
In 1872, long before women were admitted to Austrian universities, he met Henriette von Aigentler, an aspiring teacher of mathematics and physics in Graz. She was refused permission to audit lectures unofficially. Boltzmann supported her decision to appeal, which was successful. On 17 July 1876 Ludwig Boltzmann married Henriette; they had three daughters: Henriette (1880), Ida (1884) and Else (1891); and a son, Arthur Ludwig (1881). Boltzmann went back to
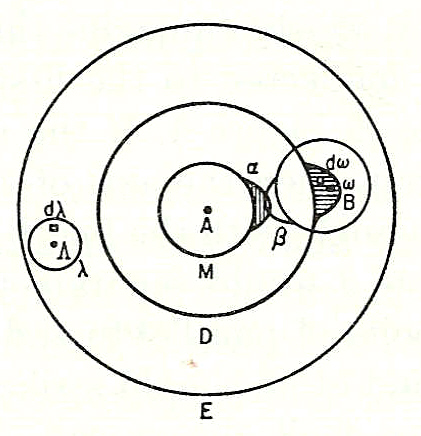 Most chemists, since the discoveries of
Most chemists, since the discoveries of
 The Boltzmann equation was developed to describe the dynamics of an ideal gas.
:
where ''ƒ'' represents the distribution function of single-particle position and momentum at a given time (see the Maxwell–Boltzmann distribution), ''F'' is a force, ''m'' is the mass of a particle, ''t'' is the time and ''v'' is an average velocity of particles.
This equation describes the temporal and
The Boltzmann equation was developed to describe the dynamics of an ideal gas.
:
where ''ƒ'' represents the distribution function of single-particle position and momentum at a given time (see the Maxwell–Boltzmann distribution), ''F'' is a force, ''m'' is the mass of a particle, ''t'' is the time and ''v'' is an average velocity of particles.
This equation describes the temporal and
 The idea that the second law of thermodynamics or "entropy law" is a law of disorder (or that dynamically ordered states are "infinitely improbable") is due to Boltzmann's view of the second law of thermodynamics.
In particular, it was Boltzmann's attempt to reduce it to a
The idea that the second law of thermodynamics or "entropy law" is a law of disorder (or that dynamically ordered states are "infinitely improbable") is due to Boltzmann's view of the second law of thermodynamics.
In particular, it was Boltzmann's attempt to reduce it to a
Chapter One: Girlhood in Vienna
gives
Ludwig Boltzmann (1844–1906).
Discusses Boltzmann's philosophical opinions, with numerous quotes. * * * {{DEFAULTSORT:Boltzmann, Ludwig 1844 births 1906 suicides Scientists from Vienna 19th-century Austrian physicists Thermodynamicists Fluid dynamicists Burials at the Vienna Central Cemetery University of Vienna alumni Members of the Royal Swedish Academy of Sciences Corresponding members of the Saint Petersburg Academy of Sciences Suicides by hanging in Austria Foreign Members of the Royal Society Foreign associates of the National Academy of Sciences Mathematical physicists Theoretical physicists Rectors of universities in Austria 19th-century Austrian philosophers 20th-century Austrian philosophers Members of the Göttingen Academy of Sciences and Humanities
philosopher
A philosopher is a person who practices or investigates philosophy. The term ''philosopher'' comes from the grc, φιλόσοφος, , translit=philosophos, meaning 'lover of wisdom'. The coining of the term has been attributed to the Greek th ...
. His greatest achievements were the development of statistical mechanics
In physics, statistical mechanics is a mathematical framework that applies statistical methods and probability theory to large assemblies of microscopic entities. It does not assume or postulate any natural laws, but explains the macroscopic be ...
, and the statistical explanation of the second law of thermodynamics. In 1877 he provided the current definition of entropy, , where Ω is the number of microstates whose energy equals the system's energy, interpreted as a measure of statistical disorder of a system.
Max Planck named the constant the Boltzmann constant.
Statistical mechanics is one of the pillars of modern physics. It describes how macroscopic observations (such as temperature and pressure) are related to microscopic parameters that fluctuate around an average. It connects thermodynamic quantities (such as heat capacity) to microscopic behavior, whereas, in classical thermodynamics, the only available option would be to measure and tabulate such quantities for various materials.
Biography
Childhood and education
Boltzmann was born in Erdberg, a suburb of Vienna. His father, Ludwig Georg Boltzmann, was a revenue official. His grandfather, who had moved to Vienna from Berlin, was a clock manufacturer, and Boltzmann's mother, Katharina Pauernfeind, was originally from Salzburg. He received his primary education at the home of his parents. Boltzmann attended high school in Linz, Upper Austria. When Boltzmann was 15, his father died. Starting in 1863, Boltzmann studiedmathematics
Mathematics is an area of knowledge that includes the topics of numbers, formulas and related structures, shapes and the spaces in which they are contained, and quantities and their changes. These topics are represented in modern mathematics ...
and physics at the University of Vienna. He received his doctorate in 1866 and his venia legendi in 1869. Boltzmann worked closely with Josef Stefan, director of the institute of physics. It was Stefan who introduced Boltzmann to Maxwell's work.
Academic career
In 1869 at age 25, thanks to aletter of recommendation
A letter of recommendation or recommendation letter, also known as a letter of reference, reference letter or simply reference, is a document in which the writer assesses the qualities, characteristics, and capabilities of the person being recommen ...
written by Josef Stefan, Boltzmann was appointed full Professor of Mathematical Physics at the University of Graz in the province of Styria
Styria (german: Steiermark ; Serbo-Croatian and sl, ; hu, Stájerország) is a state (''Bundesland'') in the southeast of Austria. With an area of , Styria is the second largest state of Austria, after Lower Austria. Styria is bordered to ...
. In 1869 he spent several months in Heidelberg working with Robert Bunsen and Leo Königsberger
Leo Königsberger (15 October 1837 – 15 December 1921) was a German mathematician, and historian of science. He is best known for his three-volume biography of Hermann von Helmholtz, which remains the standard reference on the subject.
In 20 ...
and in 1871 with Gustav Kirchhoff and Hermann von Helmholtz in Berlin. In 1873 Boltzmann joined the University of Vienna as Professor of Mathematics and there he stayed until 1876.
 In 1872, long before women were admitted to Austrian universities, he met Henriette von Aigentler, an aspiring teacher of mathematics and physics in Graz. She was refused permission to audit lectures unofficially. Boltzmann supported her decision to appeal, which was successful. On 17 July 1876 Ludwig Boltzmann married Henriette; they had three daughters: Henriette (1880), Ida (1884) and Else (1891); and a son, Arthur Ludwig (1881). Boltzmann went back to
In 1872, long before women were admitted to Austrian universities, he met Henriette von Aigentler, an aspiring teacher of mathematics and physics in Graz. She was refused permission to audit lectures unofficially. Boltzmann supported her decision to appeal, which was successful. On 17 July 1876 Ludwig Boltzmann married Henriette; they had three daughters: Henriette (1880), Ida (1884) and Else (1891); and a son, Arthur Ludwig (1881). Boltzmann went back to Graz
Graz (; sl, Gradec) is the capital city of the Austrian state of Styria and second-largest city in Austria after Vienna. As of 1 January 2021, it had a population of 331,562 (294,236 of whom had principal-residence status). In 2018, the popul ...
to take up the chair of Experimental Physics. Among his students in Graz were Svante Arrhenius and Walther Nernst
Walther Hermann Nernst (; 25 June 1864 – 18 November 1941) was a German chemist known for his work in thermodynamics, physical chemistry, electrochemistry, and solid state physics. His formulation of the Nernst heat theorem helped pave the wa ...
. He spent 14 happy years in Graz and it was there that he developed his statistical concept of nature.
Boltzmann was appointed to the Chair of Theoretical Physics at the University of Munich in Bavaria, Germany in 1890.
In 1894, Boltzmann succeeded his teacher Joseph Stefan as Professor of Theoretical Physics at the University of Vienna.
Final years and death
Boltzmann spent a great deal of effort in his final years defending his theories.Cercignani, Carlo (1998) Ludwig Boltzmann: The Man Who Trusted Atoms. Oxford University Press. He did not get along with some of his colleagues in Vienna, particularlyErnst Mach
Ernst Waldfried Josef Wenzel Mach ( , ; 18 February 1838 – 19 February 1916) was a Moravian-born Austrian physicist and philosopher, who contributed to the physics of shock waves. The ratio of one's speed to that of sound is named the Mach ...
, who became a professor of philosophy and history of sciences in 1895. That same year Georg Helm and Wilhelm Ostwald presented their position on energetics Energetics is the study of energy, and may refer to:
* Thermodynamics
* Bioenergetics
* Energy flow (ecology)
Energy flow is the flow of energy through living things within an ecosystem. All living organisms can be organized into producers and ...
at a meeting in Lübeck. They saw energy, and not matter, as the chief component of the universe. Boltzmann's position carried the day among other physicists who supported his atomic theories in the debate. In 1900, Boltzmann went to the University of Leipzig, on the invitation of Wilhelm Ostwald. Ostwald offered Boltzmann the professorial chair in physics, which became vacant when Gustav Heinrich Wiedemann
Gustav Heinrich Wiedemann (; 2 October 1826 – 24 March 1899) was a German physicist and scientific author.
Life
Wiedemann was born in Berlin the son of a merchant who died two years later. Following the death of his mother in 1842 he lived wi ...
died. After Mach retired due to bad health, Boltzmann returned to Vienna in 1902. In 1903, Boltzmann, together with Gustav von Escherich
Gustav Ritter von Escherich (1 June 1849 – 28 January 1935) was an Austrian mathematician.
Biography
Born in Mantua, he studied mathematics and physics at the University of Vienna. From 1876 to 1879 he was professor at the University of Graz. ...
and Emil Müller Emil Muller or Emil Müller may refer to:
* Emil Müller (mathematician) (1861–1927), Austrian mathematician
* Emil Müller (German officer), an officer in the World War I Imperial German Army charged with war crimes at the Leipzig War Crimes Tri ...
, founded the Austrian Mathematical Society. His students included Karl Přibram
Karl Eman Přibram (22 December 1877, Prague – 15 July 1973, Washington, D.C.), also known as “Karl Pribram”, was an Austrian-born economist. He is most noted for his work in labor economics, in industrial organization, and in the hist ...
, Paul Ehrenfest and Lise Meitner
Elise Meitner ( , ; 7 November 1878 – 27 October 1968) was an Austrian-Swedish physicist who was one of those responsible for the discovery of the element protactinium and nuclear fission. While working at the Kaiser Wilhelm Institute on rad ...
.
In Vienna, Boltzmann taught physics and also lectured on philosophy. Boltzmann's lectures on natural philosophy were very popular and received considerable attention. His first lecture was an enormous success. Even though the largest lecture hall had been chosen for it, the people stood all the way down the staircase. Because of the great successes of Boltzmann's philosophical lectures, the Emperor invited him for a reception at the Palace.
In 1906, Boltzmann's deteriorating mental condition forced him to resign his position, and his symptoms indicate he experienced what would today be diagnosed as bipolar disorder. Four months later he died by suicide on 5 September 1906, by hanging himself while on vacation with his wife and daughter in Duino, near Trieste (then Austria).
He is buried in the Viennese Zentralfriedhof. His tombstone bears the inscription of Boltzmann's entropy formula: .
Philosophy
Boltzmann'skinetic theory of gases
Kinetic (Ancient Greek: κίνησις “kinesis”, movement or to move) may refer to:
* Kinetic theory, describing a gas as particles in random motion
* Kinetic energy, the energy of an object that it possesses due to its motion
Art and enter ...
seemed to presuppose the reality of atoms and molecules, but almost all German philosophers
German(s) may refer to:
* Germany (of or related to)
** Germania (historical use)
* Germans, citizens of Germany, people of German ancestry, or native speakers of the German language
** For citizens of Germany, see also German nationality law
**Ge ...
and many scientists like Ernst Mach
Ernst Waldfried Josef Wenzel Mach ( , ; 18 February 1838 – 19 February 1916) was a Moravian-born Austrian physicist and philosopher, who contributed to the physics of shock waves. The ratio of one's speed to that of sound is named the Mach ...
and the physical chemist Wilhelm Ostwald disbelieved their existence.
Physics
Boltzmann's most important scientific contributions were inkinetic theory
Kinetic (Ancient Greek: κίνησις “kinesis”, movement or to move) may refer to:
* Kinetic theory, describing a gas as particles in random motion
* Kinetic energy, the energy of an object that it possesses due to its motion
Art and ente ...
, including for motivating the Maxwell–Boltzmann distribution as a description of molecular speeds in a gas. Maxwell–Boltzmann statistics and the Boltzmann distribution remain central in the foundations of classical statistical mechanics. They are also applicable to other phenomena
A phenomenon ( : phenomena) is an observable event. The term came into its modern philosophical usage through Immanuel Kant, who contrasted it with the noumenon, which ''cannot'' be directly observed. Kant was heavily influenced by Gottfried W ...
that do not require quantum statistics
Particle statistics is a particular description of multiple particles in statistical mechanics. A key prerequisite concept is that of a statistical ensemble (an idealization comprising the state space of possible states of a system, each labeled w ...
and provide insight into the meaning of temperature.
 Most chemists, since the discoveries of
Most chemists, since the discoveries of John Dalton
John Dalton (; 5 or 6 September 1766 – 27 July 1844) was an English chemist, physicist and meteorologist. He is best known for introducing the atomic theory into chemistry, and for his research into colour blindness, which he had. Colour b ...
in 1808, and James Clerk Maxwell in Scotland and Josiah Willard Gibbs in the United States, shared Boltzmann's belief in atoms and molecules, but much of the physics establishment did not share this belief until decades later. Boltzmann had a long-running dispute with the editor of the preeminent German physics journal of his day, who refused to let Boltzmann refer to atoms and molecules as anything other than convenient theoretical constructs. Only a couple of years after Boltzmann's death, Perrin's studies of colloid
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend ...
al suspensions (1908–1909), based on Einstein's theoretical studies of 1905, confirmed the values of the Avogadro constant and the Boltzmann constant, convincing the world that the tiny particles really exist.
To quote Planck, "The logarithmic connection between entropy and probability was first stated by L. Boltzmann in his kinetic theory of gases
Kinetic (Ancient Greek: κίνησις “kinesis”, movement or to move) may refer to:
* Kinetic theory, describing a gas as particles in random motion
* Kinetic energy, the energy of an object that it possesses due to its motion
Art and enter ...
". This famous formula for entropy ''S'' is
:
where ''k''B is the Boltzmann constant, and ln is the natural logarithm
The natural logarithm of a number is its logarithm to the base of the mathematical constant , which is an irrational and transcendental number approximately equal to . The natural logarithm of is generally written as , , or sometimes, if ...
. ''W'' is ''Wahrscheinlichkeit'', a German word meaning the probability of occurrence of a macrostate
In statistical mechanics, a microstate is a specific microscopic configuration of a thermodynamic system that the system may occupy with a certain probability in the course of its thermal fluctuations. In contrast, the macrostate of a system refe ...
or, more precisely, the number of possible microstates
A microstate or ministate is a sovereign state having a very small population or very small land area, usually both. However, the meanings of "state" and "very small" are not well-defined in international law.Warrington, E. (1994). "Lilliputs ...
corresponding to the macroscopic state of a system — the number of (unobservable) "ways" in the (observable) thermodynamic state of a system that can be realized by assigning different positions and momenta
Momenta is an autonomous driving company headquartered in Beijing, China that aims to build the 'Brains' for autonomous vehicles.
In December 2021, Momenta and BYD established a 100 million yuan ($15.7 million) joint venture to deploy autonomous ...
to the various molecules. Boltzmann's paradigm
In science and philosophy, a paradigm () is a distinct set of concepts or thought patterns, including theories, research methods, postulates, and standards for what constitute legitimate contributions to a field.
Etymology
''Paradigm'' comes f ...
was an ideal gas of ''N'' ''identical'' particles, of which ''N''''i'' are in the ''i''th microscopic condition (range) of position and momentum. ''W'' can be counted using the formula for permutations
In mathematics, a permutation of a set is, loosely speaking, an arrangement of its members into a sequence or linear order, or if the set is already ordered, a rearrangement of its elements. The word "permutation" also refers to the act or proc ...
:
where ''i'' ranges over all possible molecular conditions, and where denotes factorial
In mathematics, the factorial of a non-negative denoted is the product of all positive integers less than or equal The factorial also equals the product of n with the next smaller factorial:
\begin
n! &= n \times (n-1) \times (n-2) \t ...
. The "correction" in the denominator account for indistinguishable particles in the same condition.
Boltzmann could also be considered one of the forerunners of quantum mechanics due to his suggestion in 1877 that the energy levels of a physical system could be discrete.
Boltzmann equation
spatial
Spatial may refer to:
*Dimension
*Space
*Three-dimensional space
Three-dimensional space (also: 3D space, 3-space or, rarely, tri-dimensional space) is a geometric setting in which three values (called ''parameters'') are required to determ ...
variation of the probability distribution for the position and momentum of a density distribution of a cloud of points in single-particle phase space
In dynamical system theory, a phase space is a space in which all possible states of a system are represented, with each possible state corresponding to one unique point in the phase space. For mechanical systems, the phase space usually ...
. (See Hamiltonian mechanics.) The first term on the left-hand side represents the explicit time variation of the distribution function, while the second term gives the spatial variation, and the third term describes the effect of any force acting on the particles. The right-hand side of the equation represents the effect of collisions.
In principle, the above equation completely describes the dynamics of an ensemble of gas particles, given appropriate boundary conditions
In mathematics, in the field of differential equations, a boundary value problem is a differential equation together with a set of additional constraints, called the boundary conditions. A solution to a boundary value problem is a solution to th ...
. This first-order differential equation has a deceptively simple appearance, since ''f'' can represent an arbitrary single-particle distribution function. Also, the force
In physics, a force is an influence that can change the motion of an object. A force can cause an object with mass to change its velocity (e.g. moving from a state of rest), i.e., to accelerate. Force can also be described intuitively as a p ...
acting on the particles depends directly on the velocity distribution function ''f''. The Boltzmann equation is notoriously difficult to integrate. David Hilbert
David Hilbert (; ; 23 January 1862 – 14 February 1943) was a German mathematician, one of the most influential mathematicians of the 19th and early 20th centuries. Hilbert discovered and developed a broad range of fundamental ideas in many a ...
spent years trying to solve it without any real success.
The form of the collision term assumed by Boltzmann was approximate. However, for an ideal gas the standard Chapman–Enskog solution of the Boltzmann equation is highly accurate. It is expected to lead to incorrect results for an ideal gas only under shock wave conditions.
Boltzmann tried for many years to "prove" the second law of thermodynamics using his gas-dynamical equation — his famous H-theorem. However the key assumption he made in formulating the collision term was " molecular chaos", an assumption which breaks time-reversal symmetry as is necessary for ''anything'' which could imply the second law. It was from the probabilistic assumption alone that Boltzmann's apparent success emanated, so his long dispute with Loschmidt
Johann Josef Loschmidt (15 March 1821 – 8 July 1895), who referred to himself mostly as Josef Loschmidt (omitting his first name), was a notable Austrian scientist who performed ground-breaking work in chemistry, physics (thermodynamics, optics ...
and others over Loschmidt's paradox ultimately ended in his failure.
Finally, in the 1970s E.G.D. Cohen
Ezechiel Godert David "Eddie" Cohen (January 16, 1923– September 24, 2017) was a Dutch-American physicist and Professor Emeritus at The Rockefeller University. He is widely recognised for his contributions to statistical physics. In 2004 Cohen ...
and J. R. Dorfman proved that a systematic (power series) extension of the Boltzmann equation to high densities is mathematically impossible. Consequently, nonequilibrium statistical mechanics
In physics, statistical mechanics is a mathematical framework that applies statistical methods and probability theory to large assemblies of microscopic entities. It does not assume or postulate any natural laws, but explains the macroscopic be ...
for dense gases and liquids focuses on the Green–Kubo relations, the fluctuation theorem, and other approaches instead.
Second thermodynamics law as a law of disorder
stochastic
Stochastic (, ) refers to the property of being well described by a random probability distribution. Although stochasticity and randomness are distinct in that the former refers to a modeling approach and the latter refers to phenomena themselv ...
collision function, or law of probability following from the random collisions of mechanical particles. Following Maxwell, Boltzmann modeled gas molecules as colliding billiard balls in a box, noting that with each collision nonequilibrium velocity distributions (groups of molecules moving at the same speed and in the same direction) would become increasingly disordered leading to a final state of macroscopic uniformity and maximum microscopic disorder or the state of maximum entropy (where the macroscopic uniformity corresponds to the obliteration of all field potentials or gradients). The second law, he argued, was thus simply the result of the fact that in a world of mechanically colliding particles disordered states are the most probable. Because there are so many more possible disordered states than ordered ones, a system will almost always be found either in the state of maximum disorder – the macrostate with the greatest number of accessible microstates such as a gas in a box at equilibrium – or moving towards it. A dynamically ordered state, one with molecules moving "at the same speed and in the same direction", Boltzmann concluded, is thus "the most improbable case conceivable...an infinitely improbable configuration of energy."
Boltzmann accomplished the feat of showing that the second law of thermodynamics is only a statistical fact. The gradual disordering of energy is analogous to the disordering of an initially ordered pack of cards
A playing card is a piece of specially prepared card stock, heavy paper, thin cardboard, plastic-coated paper, cotton-paper blend, or thin plastic that is marked with distinguishing motifs. Often the front (face) and back of each card has a fi ...
under repeated shuffling, and just as the cards will finally return to their original order if shuffled a gigantic number of times, so the entire universe must some-day regain, by pure chance, the state from which it first set out. (This optimistic coda to the idea of the dying universe becomes somewhat muted when one attempts to estimate the timeline which will probably elapse before it spontaneously occurs.) The tendency for entropy increase seems to cause difficulty to beginners in thermodynamics, but is easy to understand from the standpoint of the theory of probability. Consider two ordinary dice
Dice (singular die or dice) are small, throwable objects with marked sides that can rest in multiple positions. They are used for generating random values, commonly as part of tabletop games, including dice games, board games, role-playing g ...
, with both sixes face up. After the dice are shaken, the chance of finding these two sixes face up is small (1 in 36); thus one can say that the random motion (the agitation) of the dice, like the chaotic collisions of molecules because of thermal energy, causes the less probable state to change to one that is more probable. With millions of dice, like the millions of atoms involved in thermodynamic calculations, the probability of their all being sixes becomes so vanishingly small that the system ''must'' move to one of the more probable states."Collier's Encyclopedia", Volume 22 Sylt to Uruguay, Thermodynamics, by Leo Peters, p. 275
Works
* * * * *Awards and honours
In 1885 he became a member of the Imperial Austrian Academy of Sciences and in 1887 he became the President of the University of Graz. He was elected a member of theRoyal Swedish Academy of Sciences
The Royal Swedish Academy of Sciences ( sv, Kungliga Vetenskapsakademien) is one of the Swedish Royal Academies, royal academies of Sweden. Founded on 2 June 1739, it is an independent, non-governmental scientific organization that takes special ...
in 1888 and a Foreign Member of the Royal Society (ForMemRS) in 1899. Numerous things are named in his honour.
See also
* Thermodynamics * Boltzmann brainReferences
Further reading
* Roman Sexl & John Blackmore (eds.), "Ludwig Boltzmann – Ausgewahlte Abhandlungen", (Ludwig Boltzmann Gesamtausgabe, Band 8), Vieweg, Braunschweig, 1982. * John Blackmore (ed.), "Ludwig Boltzmann – His Later Life and Philosophy, 1900–1906, Book One: A Documentary History", Kluwer, 1995. * John Blackmore, "Ludwig Boltzmann – His Later Life and Philosophy, 1900–1906, Book Two: The Philosopher", Kluwer, Dordrecht, Netherlands, 1995. * John Blackmore (ed.), "Ludwig Boltzmann – Troubled Genius as Philosopher", in Synthese, Volume 119, Nos. 1 & 2, 1999, pp. 1–232. * * Boltzmann, ''Ludwig Boltzmann – Leben und Briefe'', ed., Walter Hoeflechner, Akademische Druck- u. Verlagsanstalt. Graz, Oesterreich, 1994 * Brush, Stephen G. (ed. & tr.), Boltzmann, ''Lectures on Gas Theory'', Berkeley, California: U. of California Press, 1964 * Brush, Stephen G. (ed.), ''Kinetic Theory'', New York: Pergamon Press, 1965 * * * * * Ehrenfest, P. & Ehrenfest, T. (1911) "Begriffliche Grundlagen der statistischen Auffassung in der Mechanik", in ''Encyklopädie der mathematischen Wissenschaften mit Einschluß ihrer Anwendungen'' Band IV, 2. Teil ( F. Klein and C. Müller (eds.). Leipzig: Teubner, pp. 3–90. Translated as ''The Conceptual Foundations of the Statistical Approach in Mechanics''. New York: Cornell University Press, 1959. * * * * * * * * * English translation by Morton Masius of the 2nd ed. of ''Waermestrahlung''. Reprinted by Dover (1959) & (1991). * Sharp, Kim (2019). ''Entropy and the Tao of Counting: A Brief Introduction to Statistical Mechanics and the Second Law of Thermodynamics'' (SpringerBriefs in Physics). Springer Nature. * Reprinted: Dover (1979).External links
* * *Ruth Lewin Sime
Ruth Lewin Sime is an American author, educator and scientific researcher, best known for publishing works on history of science.'' John Simon Guggenheim Memorial Foundation website''"Ruth Lewin Sime" Accessed 06 February 2018. She has written sev ...
, ''Lise Meitner: A Life in Physics'Chapter One: Girlhood in Vienna
gives
Lise Meitner
Elise Meitner ( , ; 7 November 1878 – 27 October 1968) was an Austrian-Swedish physicist who was one of those responsible for the discovery of the element protactinium and nuclear fission. While working at the Kaiser Wilhelm Institute on rad ...
's account of Boltzmann's teaching and career.
* Eftekhari, Ali,Ludwig Boltzmann (1844–1906).
Discusses Boltzmann's philosophical opinions, with numerous quotes. * * * {{DEFAULTSORT:Boltzmann, Ludwig 1844 births 1906 suicides Scientists from Vienna 19th-century Austrian physicists Thermodynamicists Fluid dynamicists Burials at the Vienna Central Cemetery University of Vienna alumni Members of the Royal Swedish Academy of Sciences Corresponding members of the Saint Petersburg Academy of Sciences Suicides by hanging in Austria Foreign Members of the Royal Society Foreign associates of the National Academy of Sciences Mathematical physicists Theoretical physicists Rectors of universities in Austria 19th-century Austrian philosophers 20th-century Austrian philosophers Members of the Göttingen Academy of Sciences and Humanities