Aniline from Nitrobenzene.svg on:
[Wikipedia]
[Google]
[Amazon]
Aniline is an organic compound with the

 The reduction of nitrobenzene to aniline was first performed by
The reduction of nitrobenzene to aniline was first performed by
 The oxidation of aniline has been heavily investigated, and can result in reactions localized at nitrogen or more commonly results in the formation of new C-N bonds. In alkaline solution, azobenzene results, whereas arsenic acid produces the violet-coloring matter violaniline. Chromic acid converts it into
The oxidation of aniline has been heavily investigated, and can result in reactions localized at nitrogen or more commonly results in the formation of new C-N bonds. In alkaline solution, azobenzene results, whereas arsenic acid produces the violet-coloring matter violaniline. Chromic acid converts it into 
 The reaction to form
The reaction to form 2 C6H5NH2 + CH2O -> CH2(C6H4NH2)2 + H2O
The resulting diamine is the precursor to 4,4'-MDI and related diisocyanates.
 Missing in such an analysis is consideration of solvation. Aniline is, for example, more basic than ammonia in the gas phase, but ten thousand times less so in aqueous solution.
Missing in such an analysis is consideration of solvation. Aniline is, for example, more basic than ammonia in the gas phase, but ten thousand times less so in aqueous solution.
C6H5NH2 + 2 CH3OH -> C6H5N(CH3)2 + 2H2O
''N''-Methylaniline and ''N'',''N''-dimethylaniline are colorless liquids with
 Other uses include rubber processing chemicals (9%),
Other uses include rubber processing chemicals (9%), 
pp 150–1
In 1932,
International Chemical Safety Card 0011
* {{Authority control Dyes German inventions Hazardous air pollutants IARC Group 3 carcinogens Phenyl compounds
formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwee ...
C6 H5 NH2. Consisting of a phenyl group
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen ...
attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical
Commodity chemicals (or bulk commodities or bulk chemicals) are a group of chemicals that are made on a very large scale to satisfy global markets. The average prices of commodity chemicals are regularly published in the chemical trade magazines an ...
, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans.
Relative to benzene, it is electron-rich. It thus participates more rapidly in electrophilic aromatic substitution reactions. Likewise, it is also prone to oxidation: while freshly purified aniline is an almost colorless oil, exposure to air results in gradual darkening to yellow or red, due to the formation of strongly colored, oxidized impurities. Aniline can be diazotized to give a diazonium salt, which can then undergo various nucleophilic substitution reactions.
“Aniline” is ultimately from Portuguese ''anil'' which means "the indigo shrub", with suffix ''-ine'' indicating "derived substance".
Like other amines, aniline is both a base (p''K''aH = 4.6) and a nucleophile, although less so than structurally similar aliphatic amines.
Because an early source of the benzene from which they are derived was coal tar, aniline dyes are also called coal tar dyes.
Structure

Aryl-N distances
In aniline, the C−N bond length is 1.41 Å, compared to 1.47 Å for cyclohexylamine, indicating partial π-bonding between N and C. The C(aryl)-NH2 distance in anilines is highly sensitive to substituent effects. This distance is 1.34 Å in2,4,6-trinitroaniline
2,4,6-Trinitroaniline, C6H4N4O6, abbreviated as TNA and also known as picramide, a nitrated amine. Materials in this group range from slight to strong oxidizing agents. If mixed with reducing agents, including hydrides, sulfides and nitrides, the ...
vs 1.44 Å in 3-methylaniline
There are three isomers of toluidine, which are organic compounds. These isomers are ''o''-toluidine, ''m''-toluidine, and ''p''-toluidine, with the prefixed letter abbreviating, respectively, ''ortho''; ''meta''; and ''para''. All three are aryl ...
.
Pyramidalization
The amine in anilines is a slightly pyramidalized molecule, with hybridization of the nitrogen somewhere between sp3 and sp2. The nitrogen is described as having high p character. The amino group in aniline is flatter (i.e., it is a "shallower pyramid") than that in an aliphatic amine, owing to conjugation of thelone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
with the aryl substituent. The observed geometry reflects a compromise between two competing factors: 1) stabilization of the N lone pair in an orbital with significant s character favors pyramidalization (orbitals with s character are lower in energy), while 2) delocalization of the N lone pair into the aryl ring favors planarity (a lone pair in a pure p orbital gives the best overlap with the orbitals of the benzene ring π system).
Consistent with these factors, substituted anilines with electron donating groups are more pyramidalized, while those with electron withdrawing groups are more planar. In the parent aniline, the lone pair is approximately 12% s character, corresponding to sp7.3 hybridization. (For comparison, alkylamines generally have lone pairs in orbitals that are close to sp3.)
The pyramidalization angle between the C–N bond and the bisector of the H–N–H angle is 142.5°. For comparison, in more strongly pyramidal methylamine, this value is ~125°, while that of formamide has an angle of 180°.
Production
Industrial aniline production involves two steps. First, benzene isnitrated
In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters between alcohols and ...
with a concentrated mixture of nitric acid and sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
at 50 to 60 °C to yield nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5 NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor t ...
. The nitrobenzene is then hydrogenated (typically at 200–300 °C) in the presence of metal catalysts:
:Nikolay Zinin Nikolay Nikolaevich Zinin (russian: link=no, Никола́й Никола́евич Зи́нин; 25 August 1812, in Shusha – 18 February 1880, in Saint Petersburg) was a Russian organic chemist.
Life
He studied at the University of Kazan where ...
in 1842, using inorganic sulfide as a reductant ( Zinin reaction). The reduction of nitrobenzene to aniline was also performed as part of reductions by Antoine Béchamp in 1854, using iron as the reductant (Bechamp reduction
The Béchamp reduction (or Béchamp process) is a chemical reaction that converts aromatic nitro compounds to their corresponding anilines using iron as the reductant..
:
This reaction was once a major route to aniline, but catalytic hydrogenati ...
).
Aniline can alternatively be prepared from ammonia and phenol derived from the cumene process.
In commerce, three brands of aniline are distinguished: aniline oil for blue, which is pure aniline; aniline oil for red, a mixture of equimolecular quantities of aniline and ortho- and para-toluidines; and aniline oil for safranine
Safranin (Safranin O or basic red 2) is a biological stain used in histology and cytology. Safranin is used as a counterstain in some staining protocols, colouring cell nuclei red. This is the classic counterstain in both Gram stains and endospo ...
, which contains aniline and ortho- toluidine and is obtained from the distillate (échappés) of the fuchsine
Fuchsine (sometimes spelled fuchsin) or rosaniline hydrochloride is a magenta dye with chemical formula C20H19N3·HCl.
fusion.
Related aniline
derivatives
The derivative of a function is the rate of change of the function's output relative to its input value.
Derivative may also refer to:
In mathematics and economics
* Brzozowski derivative in the theory of formal languages
* Formal derivative, an ...
Many analogues of aniline are known where the phenyl group is further substituted. These include toluidines, xylidines, chloroanilines, aminobenzoic acid Aminobenzoic acid (a benzoic acid with an amino group) can refer to:
* 4-Aminobenzoic acid (''p''-aminobenzoic acid or ''para''-aminobenzoic acid)
* 3-Aminobenzoic acid (''m''-aminobenzoic acid or ''meta''-aminobenzoic acid)
* 2-aminobenzoic acid ...
s, nitroaniline The term nitroaniline in chemistry refers to a derivative of aniline (C6H5NH2) containing a nitro group
In organic chemistry, nitro compounds are organic compounds that contain one or more nitro functional groups (). The nitro group is one of ...
s, and many others. They often are prepared by nitration of the substituted aromatic compounds followed by reduction. For example, this approach is used to convert toluene into toluidines and chlorobenzene into 4-chloroaniline. Alternatively, using Buchwald-Hartwig coupling or Ullmann reaction approaches, aryl halides can be aminated with aqueous or gaseous ammonia.
Reactions
The chemistry of aniline is rich because the compound has been cheaply available for many years. Below are some classes of its reactions.Oxidation
 The oxidation of aniline has been heavily investigated, and can result in reactions localized at nitrogen or more commonly results in the formation of new C-N bonds. In alkaline solution, azobenzene results, whereas arsenic acid produces the violet-coloring matter violaniline. Chromic acid converts it into
The oxidation of aniline has been heavily investigated, and can result in reactions localized at nitrogen or more commonly results in the formation of new C-N bonds. In alkaline solution, azobenzene results, whereas arsenic acid produces the violet-coloring matter violaniline. Chromic acid converts it into quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds
, whereas uch as benzene or naphthalene
Uch ( pa, ;
ur, ), frequently referred to as Uch Sharīf ( pa, ;
ur, ; ''"Noble Uch"''), is a historic city in the southern part of Pakistan's Punjab province. Uch may have been founded as Alexandria on the Indus, a town founded by Alexand ...
by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double ...chlorate
The chlorate anion has the formula ClO3-. In this case, the chlorine atom is in the +5 oxidation state. "Chlorate" can also refer to chemical compounds containing this anion; chlorates are the salts of chloric acid. "Chlorate", when followed by ...
s, in the presence of certain metallic salts (especially of vanadium
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer ( pas ...
), give aniline black. Hydrochloric acid and potassium chlorate give chloranil. Potassium permanganate in neutral solution oxidizes it to nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5 NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor t ...
; in alkaline solution to azobenzene, ammonia, and oxalic acid
Oxalic acid is an organic acid with the systematic name ethanedioic acid and formula . It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early inve ...
; in acid solution to aniline black. Hypochlorous acid gives 4-aminophenol
4-Aminophenol (or ''para''-aminophenol or ''p''-aminophenol) is an organic compound with the formula H2NC6H4OH. Typically available as a white powder, it is commonly used as a developer for black-and-white film, marketed under the name Rodinal.
R ...
and para-amino diphenylamine. Oxidation with persulfate
A persulfate (sometimes known as peroxysulfate or peroxodisulfate) is a compound containing the anions or . The anion contains one peroxide group per sulfur center, whereas in , the peroxide group bridges the sulfur atoms. In both cases, sulfu ...
affords a variety of polyanilines. These polymers exhibit rich redox and acid-base properties.
Electrophilic reactions at ortho- and para- positions
Like phenols, aniline derivatives are highly susceptible toelectrophilic substitution
Electrophilic substitution reactions are chemical reactions in which an electrophile displaces a functional group in a compound, which is typically, but not always, aromatic. Aromatic substitution reactions are characteristic of aromatic compounds ...
reactions. Its high reactivity reflects that it is an enamine, which enhances the electron-donating ability of the ring. For example, reaction of aniline with sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular formu ...
at 180 °C produces sulfanilic acid
Sulfanilic acid (4-aminobenzenesulfonic acid) is an organic compound with the formula H3NC6H4SO3. It is an off-white solid. It is a zwitterion, which explains its high melting point. It is a common building block in organic chemistry."Sulphanili ...
, .
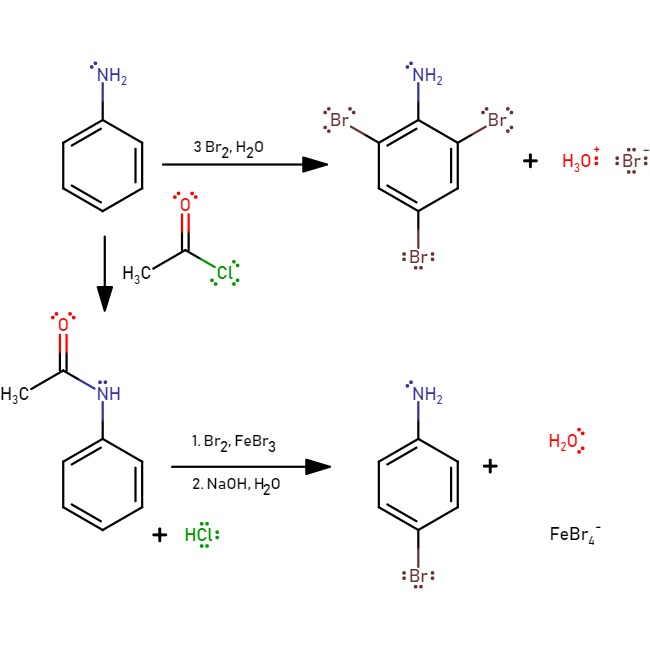
If bromine water is added to aniline, the bromine water
Bromine water is an oxidizing, intense brown mixture containing diatomic bromine (Br2) dissolved in water (H2O). It is often used as a reactive in chemical assays of recognition for substances which react with bromine in an aqueous environment wi ...
is decolourised and a white precipitate of 2,4,6-tribromoaniline is formed. To generate the mono-substituted product, a protection with acetyl chloride is required:
 The reaction to form
The reaction to form 4-bromoaniline
4-Bromoaniline is a compound where an aniline molecule is substituted with a bromine atom on the ''para'' position. Commercially available, this compound may be used as a building block, e.g. in the preparation of p-bromobiphenyl
Biphenyl (als ...
is to protect the amine with acetyl chloride, then hydrolyse back to reform aniline.
The largest scale industrial reaction of aniline involves its alkylation with formaldehyde. An idealized equation is shown:
:Reactions at nitrogen
Basicity
Aniline is a weak base. Aromatic amines such as aniline are, in general, much weaker bases than aliphatic amines. Aniline reacts with strong acids to form theanilinium
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting ...
(or phenylammonium) ion ().
Traditionally, the weak basicity of aniline is attributed to a combination of inductive effect from the more electronegative sp2 carbon and resonance effects, as the lone pair on the nitrogen is partially delocalized into the pi system of the benzene ring. (see the picture below):
Acylation
Aniline reacts withacyl chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
s such as acetyl chloride to give amides. The amides formed from aniline are sometimes called anilides, for example is acetanilide. At high temperatures aniline and carboxylic acids react to give the anilides.
''N''-Alkylation
''N''-Methylation of aniline withmethanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a ...
at elevated temperatures over acid catalysts
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequ ...
gives ''N''-methylaniline and ''N'',''N''-dimethylaniline:
:boiling point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor.
The boiling point of a liquid varies depending upon the surrounding envir ...
s of 193–195 °C and 192 °C, respectively. These derivatives are of importance in the color industry. Aniline combines directly with alkyl iodides to form secondary and tertiary amines.
Carbon disulfide derivatives
Boiled with carbon disulfide, it gives sulfocarbanilide (diphenyl thiourea) (), which may be decomposed into phenyl isothiocyanate (), and triphenyl guanidine ().Diazotization
Aniline and its ring-substituted derivatives react with nitrous acid to form diazonium salts. Through these intermediates, the amine group can be converted to a hydroxyl (),nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix ''cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including met ...
(), or halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluor ...
group (, where X is a halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
) via Sandmeyer reactions. This diazonium salt can also be reacted with and phenol to produce a dye known as benzeneazophenol, in a process called '' coupling''.
The reaction of converting primary aromatic amine into diazonium salt is called diazotisation.
In this reaction primary aromatic amine reacts with sodium nitrile and with 2 moles of HCl which is known as Ice cold mixture because the temperature use to be 0.5 °C and it forms benzene diazonium salt as major product and water and sodium chloride.
Other reactions
It reacts with nitrobenzene to produce phenazine in the Wohl-Aue reaction. Hydrogenation givescyclohexylamine
Cyclohexylamine is an organic compound, belonging to the aliphatic amine class. It is a colorless liquid, although, like many amines, samples are often colored due to contaminants. It has a fishy odor and is miscible with water. Like other amines, ...
.
Being a standard reagent in laboratories, aniline is used for many niche reactions. Its acetate is used in the aniline acetate test
The aniline acetate test is a chemical test for the presence of certain carbohydrates, in which they are converted to furfural with hydrochloric acid, which reacts with aniline acetate to produce a bright pink color. Pentoses give a strong reacti ...
for carbohydrates, identifying pentoses by conversion to furfural
Furfural is an organic compound with the formula C4H3OCHO. It is a colorless liquid, although commercial samples are often brown. It has an aldehyde group attached to the 2-position of furan. It is a product of the dehydration of sugars, as occurs ...
. It is used to stain neural RNA
Ribonucleic acid (RNA) is a polymeric molecule essential in various biological roles in coding, decoding, regulation and expression of genes. RNA and deoxyribonucleic acid ( DNA) are nucleic acids. Along with lipids, proteins, and carbohydra ...
blue in the Nissl stain
Franz Alexander Nissl (9 September 1860, in Frankenthal – 11 August 1919, in Munich) was a German psychiatrist and medical researcher. He was a noted neuropathologist.
Early life
Nissl was born in Frankenthal to Theodor Nissl and Maria Haas ...
.
Uses
Aniline is predominantly used for the preparation of methylenedianiline and related compounds by condensation with formaldehyde. The diamines are condensed withphosgene
Phosgene is the organic chemical compound with the formula COCl2. It is a toxic, colorless gas; in low concentrations, its musty odor resembles that of freshly cut hay or grass. Phosgene is a valued and important industrial building block, espe ...
to give methylene diphenyl diisocyanate, a precursor to urethane polymers.
:herbicide
Herbicides (, ), also commonly known as weedkillers, are substances used to control undesired plants, also known as weeds.EPA. February 201Pesticides Industry. Sales and Usage 2006 and 2007: Market Estimates. Summary in press releasMain page fo ...
s (2%), and dyes and pigments (2%). As additives to rubber, aniline derivatives such as phenylenediamines and diphenylamine, are antioxidants. Illustrative of the drugs prepared from aniline is paracetamol
Paracetamol, also known as acetaminophen, is a medication used to treat fever and mild to moderate pain. Common brand names include Tylenol and Panadol.
At a standard dose, paracetamol only slightly decreases body temperature; it is inferior ...
(acetaminophen, Tylenol). The principal use of aniline in the dye industry is as a precursor to indigo, the blue of blue jeans.

History
Aniline was first isolated in 1826 byOtto Unverdorben
Otto Unverdorben (13 October 1806 – 28 November 1873) was a German chemist and merchant who was born in Dahme/Marke. After completing his schooling in Dresden, he studied chemistry at Halle, Leipzig and Berlin.
In 1826 at the age of 20, Unver ...
by destructive distillation of indigo. He called it ''Crystallin''. In 1834, Friedlieb Runge isolated a substance from coal tar that turned a beautiful blue color when treated with chloride of lime. He named it ''kyanol'' or ''cyanol''. In 1840, Carl Julius Fritzsche
Carl Julius Fritzsche (17 October 1808 in Neustadt in Sachsen, Neustadt – 8 June 1871) was a German pharmacist and chemist. He was a nephew of pharmacist Friedrich Adolph August Struve (1781–1840).
After five years spent working at his unc ...
(1808–1871) treated indigo with caustic potash
Potassium hydroxide is an inorganic compound with the formula K OH, and is commonly called caustic potash.
Along with sodium hydroxide (NaOH), KOH is a prototypical strong base. It has many industrial and niche applications, most of which explo ...
and obtained an oil that he named ''aniline'', after an indigo-yielding plant, anil ('' Indigofera suffruticosa''). In 1842, Nikolay Nikolaevich Zinin reduced nitrobenzene
Nitrobenzene is an organic compound with the chemical formula C6H5 NO2. It is a water-insoluble pale yellow oil with an almond-like odor. It freezes to give greenish-yellow crystals. It is produced on a large scale from benzene as a precursor t ...
and obtained a base that he named ''benzidam''. In 1843, August Wilhelm von Hofmann showed that these were all the same substance, known thereafter as ''phenylamine'' or ''aniline''.
Synthetic dye industry
In 1856, while trying to synthesise quinine, von Hofmann's student William Henry Perkin discoveredmauveine
Mauveine, also known as aniline purple and Perkin's mauve, was one of the first synthetic dyes. It was discovered serendipitously by William Henry Perkin in 1856 while he was attempting to synthesise the phytochemical quinine for the treatment of m ...
and went into industry producing the first commercial synthetic Synthetic things are composed of multiple parts, often with the implication that they are artificial. In particular, 'synthetic' may refer to:
Science
* Synthetic chemical or compound, produced by the process of chemical synthesis
* Synthetic o ...
dye. Other aniline dyes followed, such as fuchsin
Fuchsine (sometimes spelled fuchsin) or rosaniline hydrochloride is a magenta dye with chemical formula C20H19N3·HCl.
, safranin
Safranin (Safranin O or basic red 2) is a biological stain used in histology and cytology. Safranin is used as a counterstain in some staining protocols, colouring cell nuclei red. This is the classic counterstain in both Gram stains and endospo ...
, and induline Induline is a dye of blue, bluish-red or black shades. Induline consists of a mixture of several intensely colored species, so the name is often indulines. It was one of the first synthetic dyes, discovered in 1863 by J. Dale and Heinrich Caro. The ...
. At the time of mauveine's discovery, aniline was expensive. Soon thereafter, applying a method reported in 1854 by Antoine Béchamp, it was prepared "by the ton". The Béchamp reduction enabled the evolution of a massive dye industry in Germany. Today, the name of BASF, originally ''Badische Anilin- und Soda-Fabrik'' (English: Baden Aniline and Soda Factory), now the largest chemical supplier, echoes the legacy of the synthetic dye industry, built via aniline dyes and extended via the related azo dye
Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C-N=N ...
s. The first azo dye was aniline yellow.
Developments in medicine
In the late 19th century, derivatives of aniline such as acetanilide andphenacetin
Phenacetin (acetophenetidin, ''N''-(4-ethoxyphenyl)acetamide) is a pain-relieving and fever-reducing drug, which was widely used following its introduction in 1887. It was withdrawn from medicinal use as dangerous from the 1970s (e.g., withdrawn ...
emerged as analgesic
An analgesic drug, also called simply an analgesic (American English), analgaesic (British English), pain reliever, or painkiller, is any member of the group of drugs used to achieve relief from pain (that is, analgesia or pain management). It ...
drugs, with their cardiac-suppressive side effects often countered with caffeine. During the first decade of the 20th century, while trying to modify synthetic dyes to treat African sleeping sickness
African trypanosomiasis, also known as African sleeping sickness or simply sleeping sickness, is an insect-borne parasitic infection of humans and other animals. It is caused by the species '' Trypanosoma brucei''. Humans are infected by two ty ...
, Paul Ehrlich – who had coined the term '' chemotherapy'' for his ''magic bullet
Magic bullet may refer to:
* Enchanted bullet obtained through a contract with the devil in the German folk legend Freischütz
** ''Der Freischütz'', an opera by Carl Maria von Weber based on the legend
* Magic bullet (medicine), the pharmacologi ...
'' approach to medicine – failed and switched to modifying Béchamp's atoxyl
Arsanilic acid, also known as aminophenyl arsenic acid or aminophenyl arsonic acid, is an organoarsenic compound, an amino derivative of phenylarsonic acid whose amine group is in the 4-position. A crystalline powder introduced medically in the l ...
, the first organic arsenical drug, and serendipitously obtained a treatment for syphilis
Syphilis () is a sexually transmitted infection caused by the bacterium ''Treponema pallidum'' subspecies ''pallidum''. The signs and symptoms of syphilis vary depending in which of the four stages it presents (primary, secondary, latent, an ...
– salvarsan – the first successful chemotherapy agent. Salvarsan's targeted microorganism, not yet recognized as a bacterium, was still thought to be a parasite, and medical bacteriologists, believing that bacteria were not susceptible to the chemotherapeutic approach, overlooked Alexander Fleming
Sir Alexander Fleming (6 August 1881 – 11 March 1955) was a Scottish physician and microbiologist, best known for discovering the world's first broadly effective antibiotic substance, which he named penicillin. His discovery in 1928 of w ...
's report in 1928 on the effects of penicillin
Penicillins (P, PCN or PEN) are a group of β-lactam antibiotics originally obtained from ''Penicillium'' moulds, principally '' P. chrysogenum'' and '' P. rubens''. Most penicillins in clinical use are synthesised by P. chrysogenum using ...
.D J Th Wagener, ''The History of Oncology'' (Houten: Springer, 2009)pp 150–1
In 1932,
Bayer
Bayer AG (, commonly pronounced ; ) is a German multinational corporation, multinational pharmaceutical and biotechnology company and one of the largest pharmaceutical companies in the world. Headquartered in Leverkusen, Bayer's areas of busi ...
sought medical applications of its dyes. Gerhard Domagk identified as an antibacterial a red azo dye, introduced in 1935 as the first antibacterial drug, prontosil, soon found at Pasteur Institute to be a prodrug
A prodrug is a medication or compound that, after intake, is metabolized (i.e., converted within the body) into a pharmacologically active drug. Instead of administering a drug directly, a corresponding prodrug can be used to improve how the drug ...
degraded '' in vivo'' into sulfanilamide
Sulfanilamide (also spelled sulphanilamide) is a sulfonamide antibacterial drug. Chemically, it is an organic compound consisting of an aniline derivatized with a sulfonamide group. Powdered sulfanilamide was used by the Allies in World War II ...
– a colorless intermediate for many, highly colorfast azo dyes – already with an expired patent, synthesized in 1908 in Vienna by the researcher Paul Gelmo for his doctoral research. By the 1940s, over 500 related sulfa drug
Sulfonamide is a functional group (a part of a molecule) that is the basis of several groups of drugs, which are called sulphonamides, sulfa drugs or sulpha drugs. The original antibacterial sulfonamides are synthetic (nonantibiotic) antimi ...
s were produced. Medications in high demand during World War II (1939–45), these first ''miracle drugs'', chemotherapy of wide effectiveness, propelled the American pharmaceutics industry. In 1939, at Oxford University, seeking an alternative to sulfa drugs, Howard Florey
Howard Walter Florey, Baron Florey (24 September 189821 February 1968) was an Australian pharmacologist and pathologist who shared the Nobel Prize in Physiology or Medicine in 1945 with Sir Ernst Chain and Sir Alexander Fleming for his role in ...
developed Fleming's penicillin into the first systemic antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of ...
drug, penicillin G. ( Gramicidin, developed by René Dubos at Rockefeller Institute in 1939, was the first antibiotic, yet its toxicity restricted it to topical use.) After World War II, Cornelius P. Rhoads
Cornelius Packard "Dusty" Rhoads (June 9, 1898 – August 13, 1959) was an American pathologist, oncologist, and hospital administrator who was involved in a racist scandal and subsequent whitewashing in the 1930s. Beginning in 1940, he served a ...
introduced the chemotherapeutic approach to cancer treatment.
Rocket fuel
Some early American rockets, such as the Aerobee and WAC Corporal, used a mixture of aniline and furfuryl alcohol as a fuel, with nitric acid as an oxidizer. The combination is hypergolic, igniting on contact between fuel and oxidizer. It is also dense, and can be stored for extended periods. Aniline was later replaced byhydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine ...
.Brian Burnell. 2016. http://www.nuclear-weapons.info/cde.htm#Corporal SSM
Toxicology and testing
Aniline is toxic by inhalation of the vapour, ingestion, or percutaneous absorption.Muir, GD (ed.) 1971, ''Hazards in the Chemical Laboratory'', The Royal Institute of Chemistry, London. TheIARC IARC may refer to:
* International Aerial Robotics Competition
* International Age Rating Coalition
* International Agency for Research on Cancer
* International Arctic Research Center
* Israel Amateur Radio Club
* iArc IARC may refer to:
* Internat ...
lists it in Group 3 (''not classifiable as to its carcinogenicity to humans'') due to the limited and contradictory data available. The early manufacture of aniline resulted in increased incidents of bladder cancer, but these effects are now attributed to naphthylamine Naphthylamine can refer to either of two isomeric chemical compounds:
*1-Naphthylamine
1-Naphthylamine is an aromatic amine derived from naphthalene. It can cause bladder cancer (transitional cell carcinoma). It crystallizes in colorless needles w ...
s, not anilines.
Aniline has been implicated as one possible cause of forest dieback.Krahl-Urban, B., Papke, H.E., Peters, K. (1988) ''Forest Decline: Cause-Effect Research in the United States of North America and Federal Republic of Germany''. Germany: Assessment Group for Biology, Ecology and Energy of the Julich Nuclear Research Center.
Many methods exist for the detection of aniline.''Basic Analytical Toxicology'' (1995), R. J. Flanagan, S. S. Brown, F. A. de Wolff, R. A. Braithwaite, B. Widdop: World Health Organization
Oxidative DNA damage
Exposure of rats to aniline can elicit a response that is toxic to the spleen, including atumorigenic
Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cells are transformed into cancer cells. The process is characterized by changes at the cellular, genetic, and epigenetic levels and abnorm ...
response. Rats exposed to aniline in drinking water, showed a significant increase in oxidative DNA damage
DNA repair is a collection of processes by which a cell identifies and corrects damage to the DNA molecules that encode its genome. In human cells, both normal metabolic activities and environmental factors such as radiation can cause DNA da ...
to the spleen, detected as a 2.8-fold increase in 8-hydroxy-2’-deoxyguanosine (8-OHdG) in their DNA. Although the base excision repair pathway was also activated, its activity was not sufficient to prevent the accumulation of 8-OHdG. The accumulation of oxidative DNA damages in the spleen following exposure to aniline may increase mutagenic events that underlie tumorigenesis.
Notes
References
*External links
*International Chemical Safety Card 0011
* {{Authority control Dyes German inventions Hazardous air pollutants IARC Group 3 carcinogens Phenyl compounds