|
Guanidine
Guanidine is the compound with the formula HNC(NH2)2. It is a colourless solid that dissolves in polar solvents. It is a strong base that is used in the production of plastics and explosives. It is found in urine predominantly in patients experiencing renal failure. A guanidine moiety also appears in larger organic molecules, including on the side chain of arginine. Structure Guanidine can be thought of as a nitrogenous analogue of carbonic acid. That is, the C=O group in carbonic acid is replaced by a C=NH group, and each OH is replaced by a group. Isobutene can be seen as the carbon analogue in much the same way. A detailed crystallographic analysis of guanidine was elucidated 148 years after its first synthesis, despite the simplicity of the molecule. In 2013, the positions of the hydrogen atoms and their displacement parameters were accurately determined using single-crystal neutron diffraction. Production Guanidine can be obtained from natural sources, being first isola ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicyandiamide
2-Cyanoguanidine is a nitrile derived from guanidine. It is a dimer of cyanamide, from which it can be prepared. 2-Cyanoguanidine is a colourless solid that is soluble in water, acetone, and alcohol, but not nonpolar organic solvents. Production and use 2-Cyanoguanidine is produced by treating cyanamide with base. It is produced in soil by decomposition of cyanamide. A variety of useful compounds are produced from 2-cyanoguanidine, guanidines and melamine. For example, acetoguanamine and benzoguanamine are prepared by condensation of cyanoguanidine with the nitrile: :(H2N)2C=NCN + RCN → (CNH2)2(CR)N3 Cyanoguanidine is also used as a slow fertilizer. Formerly, it was used as a fuel in some explosives. It is used in the adhesive industry as a curing agent for epoxy resins. Chemistry Two tautomeric forms exist, differing in the protonation and bonding of the nitrogen to which the nitrile group is attached. : 2-Cyanoguanidine can also exist in a zwitterionic form via a f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biguanidine
Biguanide () is the organic compound with the formula HN(C(NH)NH2)2. It is a colorless solid that dissolves in water to give highly basic solution. These solutions slowly hydrolyse to ammonia and urea. Synthesis Biguanide can be obtained from the reaction of dicyandiamide with ammonia, via a Pinner-type process. :\mathrm Biguanide was first synthesized by Bernhard Rathke in 1879. Biguanidine drugs A variety of derivatives of biguanide are used as pharmaceutical drugs. Antihyperglycemic agents The term "biguanidine" often refers specifically to a class of drugs that function as oral antihyperglycemic drug A drug is any chemical substance that causes a change in an organism's physiology or psychology when consumed. Drugs are typically distinguished from food and substances that provide nutritional support. Consumption of drugs can be via inhal ...s used for diabetes mellitus or prediabetes treatment. Examples include: * Metformin - widely used in treatment of diabete ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arginine
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) and both the amino and guanidino groups are protonated, resulting in a cation. Only the -arginine (symbol Arg or R) enantiomer is found naturally. Arg residues are common components of proteins. It is encoded by the codons CGU, CGC, CGA, CGG, AGA, and AGG. The guanidine group in arginine is the precursor for the biosynthesis of nitric oxide. Like all amino acids, it is a white, water-soluble solid. History Arginine was first isolated in 1886 from yellow lupin seedlings by the German chemist Ernst Schulze and his assistant Ernst Steiger. He named it from the Greek ''árgyros'' (ἄργυρος) meaning "silver" due to the silver-white appearance of arginine nitrate crystals. In 1897, Schulze and Ernst Winterstein (1865–1949) determined the struct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
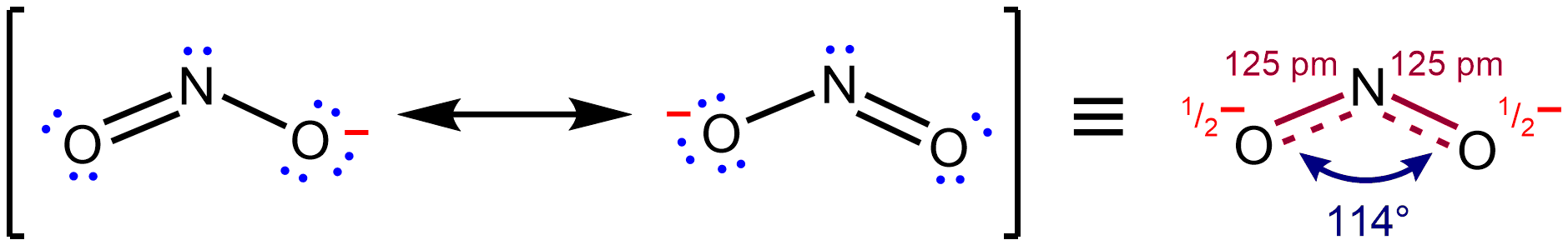
Resonance (chemistry)
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or ''canonical structures'') into a resonance hybrid (or ''hybrid structure'') in valence bond theory. It has particular value for analyzing delocalized electrons where the bonding cannot be expressed by one single Lewis structure. Overview Under the framework of valence bond theory, resonance is an extension of the idea that the bonding in a chemical species can be described by a Lewis structure. For many chemical species, a single Lewis structure, consisting of atoms obeying the octet rule, possibly bearing formal charges, and connected by bonds of positive integer order, is sufficient for describing the chemical bonding and rationalizing experimentally determined molecular properties like bond lengths, angles, and dipole moment. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid. Urea serves an important role in the metabolism of nitrogen-containing compounds by animals and is the main nitrogen-containing substance in the urine of mammals. It is a colorless, odorless solid, highly soluble in water, and practically non-toxic ( is 15 g/kg for rats). Dissolved in water, it is neither acidic nor alkaline. The body uses it in many processes, most notably nitrogen excretion. The liver forms it by combining two ammonia molecules () with a carbon dioxide () molecule in the urea cycle. Urea is widely used in fertilizers as a source of nitrogen (N) and is an important raw material for the chemical industry. In 1828 Friedrich Wöhler discovered that urea can be produced from inorganic starting materials, which was an important conceptual milesto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resonance Stabilization
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or ''canonical structures'') into a resonance hybrid (or ''hybrid structure'') in valence bond theory. It has particular value for analyzing delocalized electrons where the bonding cannot be expressed by one single Lewis structure. Overview Under the framework of valence bond theory, resonance is an extension of the idea that the bonding in a chemical species can be described by a Lewis structure. For many chemical species, a single Lewis structure, consisting of atoms obeying the octet rule, possibly bearing formal charges, and connected by bonds of positive integer order, is sufficient for describing the chemical bonding and rationalizing experimentally determined molecular properties like bond lengths, angles, and dipole moment. Howev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Explosives
An explosive (or explosive material) is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two substances. The potential energy stored in an explosive material may, for example, be * chemical energy, such as nitroglycerin or grain dust * pressurized gas, such as a gas cylinder, aerosol can, or BLEVE * nuclear energy, such as in the fissile isotopes uranium-235 and plutonium-239 Explosive materials may be categorized by the speed at which they expand. Materials that detonate (the front of the chemical reaction moves faster through the material than the speed of sound) are said to be "high explosives" and materials that deflagrate are said to be "low explosives". Explosives ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonolysis
In chemistry, ammonolysis (/am·mo·nol·y·sis/) is the process of splitting ammonia into NH2- + H+. Ammonolysis reactions can be conducted with organic compounds to produce amines (molecules containing a nitrogen atom with a lone pair, :N), or with inorganic compounds to produce nitrides. This reaction is analogous to hydrolysis in which water molecules are split. Similar to water, liquid ammonia also undergoes auto-ionization, , where the rate constant is k = 1.9*10^-38. Organic compounds such as alkyl halides, hydroxyls (hydroxyl nitriles and carbohydrates), carbonyl (aldehydes/ketones/esters/alcohols), and sulfur (sulfonyl derivatives) can all undergo ammonolysis in liquid ammonia. Organic synthesis Mechanism: ammonolysis of esters This mechanism is similar to the hydrolysis of esters, the ammonia attacks the electrophilic carbonyl carbon forming a tetrahedral intermediate. The reformation of the C-O double bond ejects the ester. The alkoxide deprotonates the ammonia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Methoxide
Sodium methoxide is the simplest sodium alkoxide. With the formula , it is a white solid, which is formed by the deprotonation of methanol. Itis a widely used reagent in industry and the laboratory. It is also a dangerously caustic base. Preparation and structure Sodium methoxide is prepared by treating methanol with sodium: : The reaction is so exothermic that ignition is possible. The resulting solution, which is colorless, is often used as a source of sodium methoxide, but the pure material can be isolated by evaporation followed by heating to remove residual methanol. As a solid, sodium methoxide is polymeric, with sheet-like arrays of centers, each bonded to four oxygen centers. The structure, and hence the basicity, of sodium methoxide in solution depends on the solvent. It is a significantly stronger base in DMSO where it is more fully ionized and free of hydrogen bonding. Applications Organic synthesis Sodium methoxide is a routinely used base in or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conjugate Acid
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid donates a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a hydrogen ion. On the other hand, a conjugate base is what is left over after an acid has donated a proton during a chemical reaction. Hence, a conjugate base is a species formed by the removal of a proton from an acid, as in the reverse reaction it is able to gain a hydrogen ion. Because some acids are capable of releasing multiple protons, the conjugate base of an acid may itself be acidic. In summary, this can be represented as the following chemical reaction: :acid + base conjugate\ base + conjugate\ acid Johannes Nicolaus Brønsted and Martin Lowry introduced the Brønsted–Lowry theory, which proposed that any compound that can transfer a proton to any other compound is an acid, and the compound that accepts the proton is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elementary Charge
The elementary charge, usually denoted by is the electric charge carried by a single proton or, equivalently, the magnitude of the negative electric charge carried by a single electron, which has charge −1 . This elementary charge is a fundamental physical constant. In the SI system of units, the value of the elementary charge is exactly defined as e = coulombs, or 160.2176634 zeptocoulombs (zC). Since the 2019 redefinition of SI base units, the seven SI base units are defined by seven fundamental physical constants, of which the elementary charge is one. In the centimetre–gram–second system of units (CGS), the corresponding quantity is . Robert A. Millikan and Harvey Fletcher's oil drop experiment first directly measured the magnitude of the elementary charge in 1909, differing from the modern accepted value by just 0.6%. Under assumptions of the then-disputed atomic theory, the elementary charge had also been indirectly inferred to ~3% accuracy f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |