|
Chloranil
Chloranil is a quinone with the molecular formula C6Cl4O2. Also known as tetrachloro-1,4-benzoquinone, it is a yellow solid. Like the parent benzoquinone, chloranil is a planar molecule J.-M. Lü, S. V. Rosokha, I. S. Neretin and J. K. Kochi, "Quinones as Electron Acceptors. X-Ray Structures, Spectral (EPR, UV-vis) Characteristics and Electron-Transfer Reactivities of Their Reduced Anion Radicals as Separated vs Contact Ion Pairs" Journal of the American Chemical Society 2006 128, 16708-16719. that functions as a mild oxidant. Synthesis and use as reagent Chloranil is produced by chlorination of phenol to give hexachlorocyclohexa-2,5-dien-1-one ("hexachlorophenol"). Hydrolysis of the dichloromethylene group in this dienone gives chloranil: :C6H5OH + 6 Cl2 → C6Cl6O + 6 HCl :C6Cl6O + H2O → C6Cl4O2 + 2 HCl Chloroanil serves as a hydrogen acceptor. It is more electrophilic than quinone itself. It is used for the aromatization reactions, such as the conversion of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloranilic Acid
Chloranilic acid is an organic compound with the chemical formula . It is a red-orange solid. The compound is obtained by hydrolysis of chloranil: : It is centrosymmetric, planar molecule. It also crystallizes as a dihydrate. Chloranilic acid is a noteworthy hydroxyquinone that is somewhat acidic owing to the presence of the two chloride substituents. The conjugate base, C6H2Cl2O42- readily forms coordination complexes often linking pairs of many metal ions. See also * Chloranil Chloranil is a quinone with the molecular formula C6Cl4O2. Also known as tetrachloro-1,4-benzoquinone, it is a yellow solid. Like the parent benzoquinone, chloranil is a planar molecule J.-M. Lü, S. V. Rosokha, I. S. Neretin and J. K. Kochi, "Qui ... References {{ketone-stub Chloroarenes 1,4-Benzoquinones Hydroxybenzoquinones Hydroquinones ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
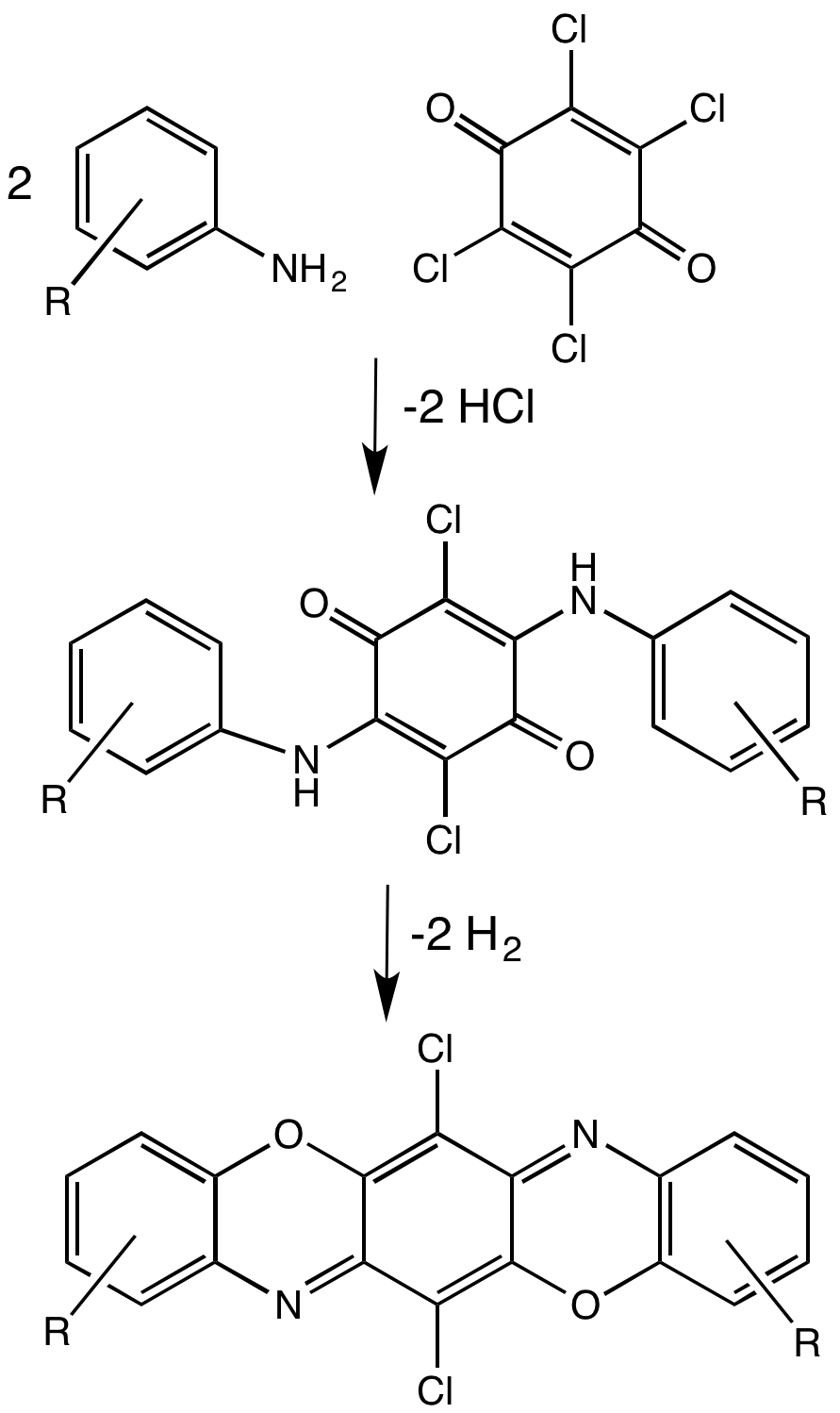
Pigment Violet 23
Pigment violet 23 is an organic compound that is a commercial pigment. It is member of the dioxazine family of heterocyclic compounds, but derived from carbazoles. It is prepared by condensation of chloranil and 3-amino-''N''-ethylcarbazole. It has a centrosymmetric angular structure. For many years, the structure was assigned, incorrectly, as having a "linear structure" ( EC no. 228-767-9, CAS RN 6358-30-1) which differ in terms of the carbazole ring fusion. Pigment violet 23 is prepared by condensation of an aniline with chloranil Chloranil is a quinone with the molecular formula C6Cl4O2. Also known as tetrachloro-1,4-benzoquinone, it is a yellow solid. Like the parent benzoquinone, chloranil is a planar molecule J.-M. Lü, S. V. Rosokha, I. S. Neretin and J. K. Kochi, "Qui ....Horst Tappe, Walter Helmling, Peter Mischke, Karl Rebsamen, Uwe Reiher, Werner Russ, Ludwig Schläfer and Petra Vermehren "Reactive Dyes"in Ullmann's Encyclopedia of Industrial Chemistry 2000, Wiley-V ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds benzene.html" ;"title="uch as benzene">uch as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds", resulting in "a fully Conjugated system, conjugated cyclic diketone, dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone" (thus the name of the class). Other important examples are 1,2-benzoquinone (''ortho''-quinone), 1,4-naphthoquinone and 9,10-anthraquinone. The name is derived from that of quinic acid (with the suffix "-one" indicating a ketone), since it is one of the compounds obtained upon oxidation of quinic acid. Quinic acid, like quinine is obtained from cinchona bark, called quinaquina in the indigenous languages of Peruvian tribes. Properties Quinones are oxidized derivatives of aromatic compounds an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexachlorocyclohexa-2,5-dien-1-one
Hexachlorocyclohexa-2,5-dien-1-one, sometimes informally called hexachlorophenol (HCP), is an organochlorine compound. It can be prepared from phenol. Despite the informal name, the compound is not a phenol but is a ketone. The informal name is derived from its method of preparation which includes phenol as a reagent. Preparation HCP is normally produced by chlorination of phenol by chlorine in the presence of metal chloride catalyst, such as ferric chloride. It can also be produced by alkaline hydrolysis of polychlorinated benzenes at high temperature and pressure, by conversion of diazonium salts of chlorinated anilines, or by chlorination of phenolsulfonic acids and benzenesulfonic acids followed by removal of the sulfonic acid group. The hydrolysis of HCP gives chloranil.{{Ullmann , author=François Muller , author2=Liliane Caillard , title=Chlorophenols, year=2011, doi=10.1002/14356007.a07_001.pub2 References See also * Pentachlorophenol * Hexachlorobenzene Hexachloroben ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone
2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (or DDQ) is the chemical reagent with formula C6Cl2(CN)2O2. This oxidant is useful for the dehydrogenation of alcohols, phenols, and steroid ketones. DDQ decomposes in water, but is stable in aqueous mineral acid. Preparation Synthesis of DDQ involves cyanation of chloranil. J. Thiele and F. Günther first reported a 6-step preparation in 1906. The substance did not receive interest until its potential as a dehydrogenation agent was discovered. A single-step chlorination from 2,3-dicyanohydroquinone was reported in 1965. Reactions The reagent removes pairs of H atoms from organic molecules. The stoichiometry of its action is illustrated by the conversion of tetralin to naphthalene: :2 C6Cl2(CN)2O2 + C10H12 → 2 C6Cl2(CN)2(OH)2 + C10H8 The resulting hydroquinone is poorly soluble in typical reaction solvents (dioxane, benzene, alkanes), which facilitates workup. Solutions of DDQ in benzene are red, due to the formation of a charge-t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sigma-Aldrich
Sigma-Aldrich (formally MilliporeSigma) is an American chemical, life science, and biotechnology company owned by the multinational chemical conglomerate Merck Group. Sigma-Aldrich was created in 1975 by the merger of Sigma Chemical Company and Aldrich Chemical Company. It grew through various acquisitions until it had over 9,600 employees and was listed on the Fortune 1000. The company has two United States headquarters, in St. Louis and Burlington, MA and has operations in approximately 40 countries. In 2015, the multinational chemical conglomerate Merck Group acquired Sigma-Aldrich for $17 billion. The company is currently a part of Merck's life science business and in combination with Merck's earlier acquired Millipore Corporation, Millipore, operates as MilliporeSigma. It is headquartered in Burlington, Massachusetts, United States. History Sigma Chemical Company of St. Louis and Aldrich Chemical Company of Milwaukee were both American specialty chemical companies when they ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG). Proline is the only proteinogenic amino acid which is a secondary amine, as the nitrogen atom is attached both to the α-carbon and to a chain of three carbons that together form a five-membered ring. History and etymology Proline was first isolated in 1900 by Richard Willstätter who obtained the amino a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organochlorides
Organochlorine chemistry is concerned with the properties of organochlorine compounds, or organochlorides, organic compounds that contain one or more carbon–chlorine bonds. The chloroalkane class (alkanes with one or more hydrogens substituted by chlorine) includes common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names, applications, and properties. Organochlorine compounds have wide use in many applications, though some are of profound environmental concern, with TCDD being one of the most notorious. Organochlorides such as trichloroethylene, tetrachloroethylene, dichloromethane and chloroform are commonly used as solvents and are referred to as "chlorinated solvents". Physical and chemical properties Chlorination modifies the physical properties of hydrocarbons in several ways. These compounds are typically denser than water due to the higher atomic weight of chlorine versus hydrogen. They have hig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fungicides
Fungicides are pesticides used to kill parasitic fungi or their spores. Fungi can cause serious damage in agriculture, resulting in losses of yield and quality. Fungicides are used both in agriculture and to fight fungal infections in animals, including humans. Fungicides are also used to control oomycetes, which are not taxonomically/genetically fungi, although sharing similar methods of infecting plants. Fungicides can either be contact, translaminar or systemic. Contact fungicides are not taken up into the plant tissue and protect only the plant where the spray is deposited. Translaminar fungicides redistribute the fungicide from the upper, sprayed leaf surface to the lower, unsprayed surface. Systemic fungicides are taken up and redistributed through the xylem vessels. Few fungicides move to all parts of a plant. Some are locally systemic, and some move upward. Most fungicides that can be bought retail are sold in liquid form, the active ingredient being present at 0.08% i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |