|
Nanofiltration
Nanofiltration is a membrane filtration process used most often to soften and disinfect water. Overview Nanofiltration is a Membrane technology, membrane filtration-based method that uses nanometer sized pores through which particles smaller than 10 nanometers pass through the membrane. Nanofiltration membranes have pore sizes from 1-10 nanometers, smaller than that used in microfiltration and ultrafiltration, but a little bit bigger than that in reverse osmosis. Membranes used are predominantly created from polymer thin films. Materials that are commonly used include polyethylene terephthalate or metals such as aluminum. Pore dimensions are controlled by pH, temperature and time during development with pore densities ranging from 1 to 106 pores per cm2. Membranes made from polyethylene terephthalate and other similar materials, are referred to as "track-etch" membranes, named after the way the pores on the membranes are made. "Tracking" involves bombarding the polymer thin fi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microfiltration
Microfiltration is a type of physical filtration process where a contaminated fluid is passed through a special pore-sized membrane filter to separate microorganisms and suspended particles from process liquid. It is commonly used in conjunction with various other separation processes such as ultrafiltration and reverse osmosis to provide a product stream which is free of undesired contaminants. General principles Microfiltration usually serves as a pre-treatment for other separation processes such as ultrafiltration, and a post-treatment for granular media filtration. The typical particle size used for microfiltration ranges from about 0.1 to 10 μm.Baker, R 2012, ''Microfiltration, in Membrane Technology and Applications'', 3rd edn, John Wiley & Sons Ltd, California. p. 303 In terms of approximate molecular weight these membranes can separate macromolecules of molecular weights generally less than 100,000 g/mol.Microfiltration/Ultrafiltration, 2008, Hyflux Membranes, accesse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Membrane Technology
Membrane technology encompasses the scientific processes used in the construction and application of membranes. Membranes are used to facilitate the transport or rejection of substances between mediums, and the mechanical separation of gas and liquid streams. In the simplest case, filtration is achieved when the pores of the membrane are smaller than the diameter of the undesired substance, such as a harmful microorganism. Membrane technology is commonly used in industries such as water treatment, chemical and metal processing, pharmaceuticals, biotechnology, the food industry, as well as the removal of environmental pollutants. After membrane construction, there is a need to characterize the prepared membrane to know more about its parameters, like pore size, function group, material properties etc, which are difficult to determine in advance. In this process, instruments such as the Scanning Electron Microscope, the Transmission electron Microscope, the Fourier Transform Infrar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reverse Osmosis
Reverse osmosis (RO) is a water purification process that uses a partially permeable membrane to separate ions, unwanted molecules and larger particles from drinking water. In reverse osmosis, an applied pressure is used to overcome osmotic pressure, a colligative property that is driven by chemical potential differences of the solvent, a thermodynamic parameter. Reverse osmosis can remove many types of dissolved and suspended chemical species as well as biological ones (principally bacteria) from water, and is used in both industrial processes and the production of potable water. The result is that the solute is retained on the pressurized side of the membrane and the pure solvent is allowed to pass to the other side. To be "selective", this membrane should not allow large molecules or ions through the wiktionary:pore, pores (holes), but should allow smaller components of the solution (such as solvent molecules, e.g., water, H2O) to pass freely. In the normal osmosis process, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Softening
Water softening is the removal of calcium, magnesium, and certain other metal cations in hard water. The resulting soft water requires less soap for the same cleaning effort, as soap is not wasted bonding with calcium ions. Soft water also extends the lifetime of plumbing by reducing or eliminating scale build-up in pipes and fittings. Water softening is usually achieved using lime softening or ion-exchange resins but is increasingly being accomplished using nanofiltration or reverse osmosis membranes. Rationale The presence of certain metal ions like calcium and magnesium, principally as bicarbonates, chlorides, and sulfates, in water causes a variety of problems. Hard water leads to the buildup of limescale, which can foul plumbing, and promote galvanic corrosion. In industrial scale water softening plants, the effluent flow from the re-generation process can precipitate scale that can interfere with sewage systems. The slippery feeling associated with washing in soft water ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Donnan Equilibrium
{{disambig ...
Donnan can refer to: *Donnan (surname) * Donnan, Iowa, a community in the United States * Donnán of Eigg, Gaelic priest of the 7th century * Donnan equilibrium ** Donnan potential Donnan potential is the difference in the Galvani potentials which appears as a result of Donnan equilibrium, named after Frederick G. Donnan, which refers to the distribution of ion species between two ionic solutions separated by a semipermea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Permeation
In physics and engineering, permeation (also called imbuing) is the penetration of a permeate (a fluid such as a liquid, gas, or vapor) through a solid. It is directly related to the concentration gradient of the permeate, a material's intrinsic permeability, and the materials' mass diffusivity. Permeation is modeled by equations such as Fick's laws of diffusion, and can be measured using tools such as a minipermeameter. Description The process of permeation involves the diffusion of molecules, called the permeant, through a membrane or interface. Permeation works through diffusion; the permeant will move from high concentration to low concentration across the interface. A material can be semipermeable, with the presence of a semipermeable membrane. Only molecules or ions with certain properties will be able to diffuse across such a membrane. This is a very important mechanism in biology where fluids inside a blood vessel need to be regulated and controlled. Permeation can occ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solutes
In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. If the attractive forces between the solvent and solute particles are greater than the attractive forces holding the solute particles together, the solvent particles pull the solute particles apart and surround them. These surrounded solute particles then move away from the solid solute and out into the solution. The mixing process of a solution happens at a scale where the effects of chemical polarity are involved, resulting in interactions that are specific to solvation. The solution usually has the state of the solvent when the solvent is the larger fraction of the mixture, as is commonly the case. One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term "aqueous solution" is used when ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shear (fluid)
Shear stress, often denoted by (Greek: tau), is the component of stress coplanar with a material cross section. It arises from the shear force, the component of force vector parallel to the material cross section. ''Normal stress'', on the other hand, arises from the force vector component perpendicular to the material cross section on which it acts. General shear stress The formula to calculate average shear stress is force per unit area.: : \tau = , where: : = the shear stress; : = the force applied; : = the cross-sectional area of material with area parallel to the applied force vector. Other forms Wall shear stress Wall shear stress expresses the retarding force (per unit area) from a wall in the layers of a fluid flowing next to the wall. It is defined as: \tau_w:=\mu\left(\frac\right)_ Where \mu is the dynamic viscosity, u the flow velocity and y the distance from the wall. It is used, for example, in the description of arterial blood flow in which case which there ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
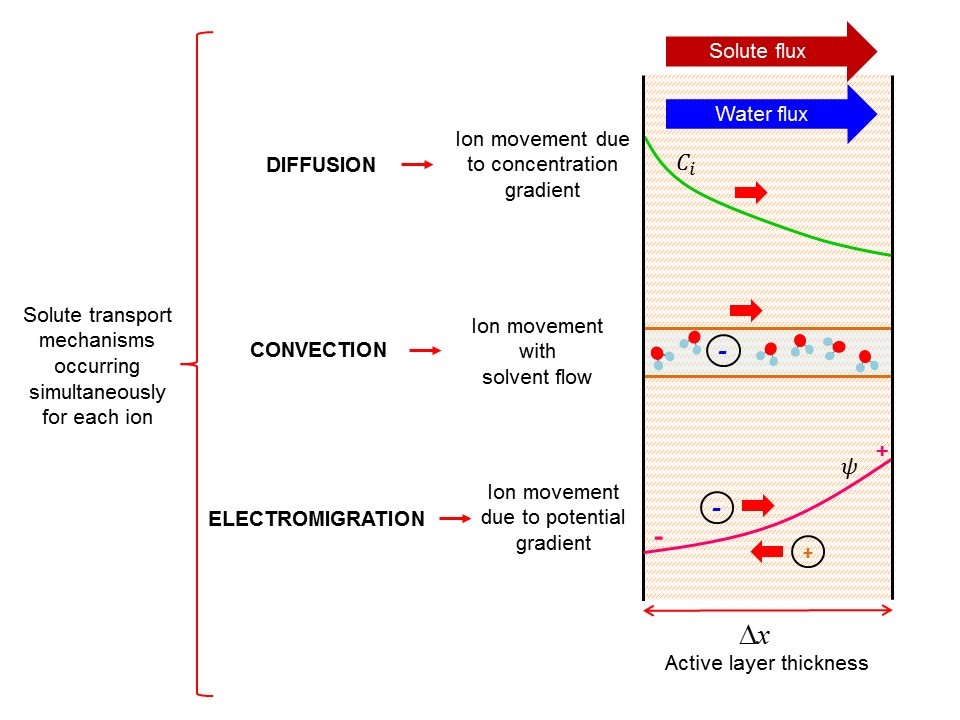
Diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical potential. It is possible to diffuse "uphill" from a region of lower concentration to a region of higher concentration, like in spinodal decomposition. The concept of diffusion is widely used in many fields, including physics (particle diffusion), chemistry, biology, sociology, economics, and finance (diffusion of people, ideas, and price values). The central idea of diffusion, however, is common to all of these: a substance or collection undergoing diffusion spreads out from a point or location at which there is a higher concentration of that substance or collection. A gradient is the change in the value of a quantity, for example, concentration, pressure, or temperature with the change in another variable, usually distance. A change in c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eddy (fluid Dynamics)
In fluid dynamics, an eddy is the swirling of a fluid and the reverse current created when the fluid is in a turbulent flow regime. The moving fluid creates a space devoid of downstream-flowing fluid on the downstream side of the object. Fluid behind the obstacle flows into the void creating a swirl of fluid on each edge of the obstacle, followed by a short reverse flow of fluid behind the obstacle flowing upstream, toward the back of the obstacle. This phenomenon is naturally observed behind large emergent rocks in swift-flowing rivers. An eddy is a movement of fluid that deviates from the general flow of the fluid. An example for an eddy is a vortex which produces such deviation. However, there are other types of eddies that are not simple vortices. For example, a Rossby wave is an eddy which is an undulation that is a deviation from mean flow, but doesn't have the local closed streamlines of a vortex. Swirl and eddies in engineering The propensity of a fluid to swirl is used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pigging
In pipeline transportation, pigging is the practice of using pipeline inspection gauges or gadgets, devices generally referred to as pigs or scrapers, to perform various maintenance operations. This is done without stopping the flow of the product in the pipeline. These operations include but are not limited to cleaning and inspecting the pipeline. This is accomplished by inserting the pig into a "pig launcher" (or "launching station") — an oversized section in the pipeline, reducing to the normal diameter. The launching station is then closed and the pressure-driven flow of the product in the pipeline is used to push the pig along the pipe until it reaches the receiving trap — the "pig catcher" (or "receiving station"). Applications Pigging is used to clean large diameter pipelines in the oil industry. Today, however, the use of smaller diameter pigging systems is now increasing in many continuous and batch process plants as plant operators search for increa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drinking Water
Drinking water is water that is used in drink or food preparation; potable water is water that is safe to be used as drinking water. The amount of drinking water required to maintain good health varies, and depends on physical activity level, age, health-related issues, and environmental conditions. This 2004 article focuses on the USA context and uses data collected from the US military. Recent work showed that the most important driver of water turnover which is closely linked to water requirements is energy expenditure. For those who work in a hot climate, up to a day may be required. Typically in developed countries, tap water meets drinking water quality standards, even though only a small proportion is actually consumed or used in food preparation. Other typical uses for tap water include washing, toilets, and irrigation. Greywater may also be used for toilets or irrigation. Its use for irrigation however may be associated with risks. Water may also be unacceptable due to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |