Diffusion on:
[Wikipedia]
[Google]
[Amazon]

 Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical potential. It is possible to diffuse "uphill" from a region of lower concentration to a region of higher concentration, like in spinodal decomposition.
The concept of diffusion is widely used in many fields, including physics ( particle diffusion), chemistry, biology, sociology, economics, and finance (diffusion of people, ideas, and price values). The central idea of diffusion, however, is common to all of these: a substance or collection undergoing diffusion spreads out from a point or location at which there is a higher concentration of that substance or collection.
A gradient is the change in the value of a quantity, for example, concentration, pressure, or
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical potential. It is possible to diffuse "uphill" from a region of lower concentration to a region of higher concentration, like in spinodal decomposition.
The concept of diffusion is widely used in many fields, including physics ( particle diffusion), chemistry, biology, sociology, economics, and finance (diffusion of people, ideas, and price values). The central idea of diffusion, however, is common to all of these: a substance or collection undergoing diffusion spreads out from a point or location at which there is a higher concentration of that substance or collection.
A gradient is the change in the value of a quantity, for example, concentration, pressure, or
 The concept of diffusion is widely used in: physics ( particle diffusion), chemistry, biology, sociology, economics, and finance (diffusion of people, ideas and of price values). However, in each case the substance or collection undergoing diffusion is "spreading out" from a point or location at which there is a higher concentration of that substance or collection.
There are two ways to introduce the notion of ''diffusion'': either a phenomenological approach starting with Fick's laws of diffusion and their mathematical consequences, or a physical and atomistic one, by considering the '' random walk of the diffusing particles''.
In the phenomenological approach, ''diffusion is the movement of a substance from a region of high concentration to a region of low concentration without bulk motion''. According to Fick's laws, the diffusion flux is proportional to the negative gradient of concentrations. It goes from regions of higher concentration to regions of lower concentration. Sometime later, various generalizations of Fick's laws were developed in the frame of thermodynamics and
The concept of diffusion is widely used in: physics ( particle diffusion), chemistry, biology, sociology, economics, and finance (diffusion of people, ideas and of price values). However, in each case the substance or collection undergoing diffusion is "spreading out" from a point or location at which there is a higher concentration of that substance or collection.
There are two ways to introduce the notion of ''diffusion'': either a phenomenological approach starting with Fick's laws of diffusion and their mathematical consequences, or a physical and atomistic one, by considering the '' random walk of the diffusing particles''.
In the phenomenological approach, ''diffusion is the movement of a substance from a region of high concentration to a region of low concentration without bulk motion''. According to Fick's laws, the diffusion flux is proportional to the negative gradient of concentrations. It goes from regions of higher concentration to regions of lower concentration. Sometime later, various generalizations of Fick's laws were developed in the frame of thermodynamics and
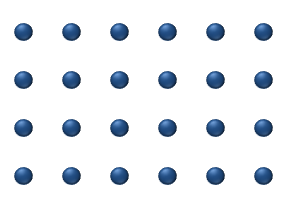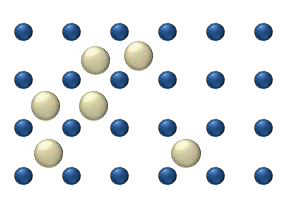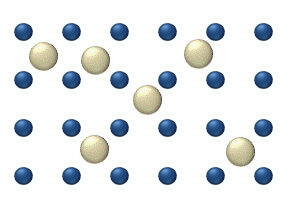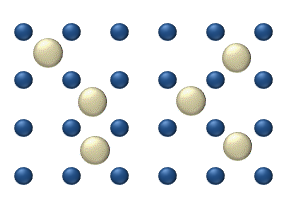 Diffusion of reagents on the surface of a catalyst may play an important role in heterogeneous catalysis. The model of diffusion in the ideal monolayer is based on the jumps of the reagents on the nearest free places. This model was used for CO on Pt oxidation under low gas pressure.
The system includes several reagents on the surface. Their surface concentrations are The surface is a lattice of the adsorption places. Each
reagent molecule fills a place on the surface. Some of the places are free. The concentration of the free places is . The sum of all (including free places) is constant, the density of adsorption places ''b''.
The jump model gives for the diffusion flux of (''i'' = 1, ..., ''n''):
:
The corresponding diffusion equation is:
:
Due to the conservation law, and we
have the system of ''m'' diffusion equations. For one component we get Fick's law and linear equations because . For two and more components the equations are nonlinear.
If all particles can exchange their positions with their closest neighbours then a simple generalization gives
:
Diffusion of reagents on the surface of a catalyst may play an important role in heterogeneous catalysis. The model of diffusion in the ideal monolayer is based on the jumps of the reagents on the nearest free places. This model was used for CO on Pt oxidation under low gas pressure.
The system includes several reagents on the surface. Their surface concentrations are The surface is a lattice of the adsorption places. Each
reagent molecule fills a place on the surface. Some of the places are free. The concentration of the free places is . The sum of all (including free places) is constant, the density of adsorption places ''b''.
The jump model gives for the diffusion flux of (''i'' = 1, ..., ''n''):
:
The corresponding diffusion equation is:
:
Due to the conservation law, and we
have the system of ''m'' diffusion equations. For one component we get Fick's law and linear equations because . For two and more components the equations are nonlinear.
If all particles can exchange their positions with their closest neighbours then a simple generalization gives
:
 Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical potential. It is possible to diffuse "uphill" from a region of lower concentration to a region of higher concentration, like in spinodal decomposition.
The concept of diffusion is widely used in many fields, including physics ( particle diffusion), chemistry, biology, sociology, economics, and finance (diffusion of people, ideas, and price values). The central idea of diffusion, however, is common to all of these: a substance or collection undergoing diffusion spreads out from a point or location at which there is a higher concentration of that substance or collection.
A gradient is the change in the value of a quantity, for example, concentration, pressure, or
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical potential. It is possible to diffuse "uphill" from a region of lower concentration to a region of higher concentration, like in spinodal decomposition.
The concept of diffusion is widely used in many fields, including physics ( particle diffusion), chemistry, biology, sociology, economics, and finance (diffusion of people, ideas, and price values). The central idea of diffusion, however, is common to all of these: a substance or collection undergoing diffusion spreads out from a point or location at which there is a higher concentration of that substance or collection.
A gradient is the change in the value of a quantity, for example, concentration, pressure, or temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measurement, measured with a thermometer.
Thermometers are calibrated in various Conversion of units of temperature, temp ...
with the change in another variable, usually distance. A change in concentration over a distance is called a concentration gradient, a change in pressure over a distance is called a pressure gradient
In atmospheric science, the pressure gradient (typically of air but more generally of any fluid) is a physical quantity that describes in which direction and at what rate the pressure increases the most rapidly around a particular location. The p ...
, and a change in temperature over a distance is called a temperature gradient.
The word ''diffusion'' derives from the Latin
Latin (, or , ) is a classical language belonging to the Italic branch of the Indo-European languages. Latin was originally a dialect spoken in the lower Tiber area (then known as Latium) around present-day Rome, but through the power of the ...
word, ''diffundere'', which means "to spread out."
A distinguishing feature of diffusion is that it depends on particle random walk, and results in mixing or mass transport without requiring directed bulk motion. Bulk motion, or bulk flow, is the characteristic of advection. The term convection is used to describe the combination of both transport phenomena
In engineering, physics, and chemistry, the study of transport phenomena concerns the exchange of mass, energy, charge, momentum and angular momentum between observed and studied systems. While it draws from fields as diverse as continuum mecha ...
.
If a diffusion process can be described by Fick's laws, it's called a normal diffusion (or Fickian diffusion); Otherwise, it's called an anomalous diffusion
Anomalous diffusion is a diffusion process with a non-linear relationship between the mean squared displacement (MSD), \langle r^(\tau )\rangle , and time. This behavior is in stark contrast to Brownian motion, the typical diffusion process descri ...
(or non-Fickian diffusion).
When talking about the extent of diffusion, two length scales are used in two different scenarios:
# Brownian motion
Brownian motion, or pedesis (from grc, πήδησις "leaping"), is the random motion of particles suspended in a medium (a liquid or a gas).
This pattern of motion typically consists of random fluctuations in a particle's position insi ...
of an impulsive point source (for example, one single spray of perfume)—the square root of the mean squared displacement from this point. In Fickian diffusion, this is , where is the dimension of this Brownian motion;
# Constant concentration source in one dimension—the diffusion length. In Fickian diffusion, this is .
Diffusion vs. bulk flow
"Bulk flow" is the movement/flow of an entire body due to a pressure gradient (for example, water coming out of a tap). "Diffusion" is the gradual movement/dispersion of concentration within a body, due to a concentration gradient, with no net movement of matter. An example of a process where both bulk motion and diffusion occur is human breathing. First, there is a "bulk flow" process. The lungs are located in thethoracic cavity
The thoracic cavity (or chest cavity) is the chamber of the body of vertebrates that is protected by the thoracic wall (rib cage and associated skin, muscle, and fascia). The central compartment of the thoracic cavity is the mediastinum. There ...
, which expands as the first step in external respiration. This expansion leads to an increase in volume of the alveoli in the lungs, which causes a decrease in pressure in the alveoli. This creates a pressure gradient between the air
The atmosphere of Earth is the layer of gases, known collectively as air, retained by Earth's gravity that surrounds the planet and forms its planetary atmosphere. The atmosphere of Earth protects life on Earth by creating pressure allowing f ...
outside the body at relatively high pressure and the alveoli at relatively low pressure. The air moves down the pressure gradient through the airways of the lungs and into the alveoli until the pressure of the air and that in the alveoli are equal, that is, the movement of air by bulk flow stops once there is no longer a pressure gradient.
Second, there is a "diffusion" process. The air arriving in the alveoli has a higher concentration of oxygen than the "stale" air in the alveoli. The increase in oxygen concentration creates a concentration gradient for oxygen between the air in the alveoli and the blood in the capillaries
A capillary is a small blood vessel from 5 to 10 micrometres (μm) in diameter. Capillaries are composed of only the tunica intima, consisting of a thin wall of simple squamous endothelial cells. They are the smallest blood vessels in the body: ...
that surround the alveoli. Oxygen then moves by diffusion, down the concentration gradient, into the blood. The other consequence of the air arriving in alveoli is that the concentration of carbon dioxide in the alveoli decreases. This creates a concentration gradient for carbon dioxide to diffuse from the blood into the alveoli, as fresh air has a very low concentration of carbon dioxide compared to the blood in the body.
Third, there is another "bulk flow" process. The pumping action of the heart then transports the blood around the body. As the left ventricle of the heart contracts, the volume decreases, which increases the pressure in the ventricle. This creates a pressure gradient between the heart and the capillaries, and blood moves through blood vessels by bulk flow down the pressure gradient.
Diffusion in the context of different disciplines
 The concept of diffusion is widely used in: physics ( particle diffusion), chemistry, biology, sociology, economics, and finance (diffusion of people, ideas and of price values). However, in each case the substance or collection undergoing diffusion is "spreading out" from a point or location at which there is a higher concentration of that substance or collection.
There are two ways to introduce the notion of ''diffusion'': either a phenomenological approach starting with Fick's laws of diffusion and their mathematical consequences, or a physical and atomistic one, by considering the '' random walk of the diffusing particles''.
In the phenomenological approach, ''diffusion is the movement of a substance from a region of high concentration to a region of low concentration without bulk motion''. According to Fick's laws, the diffusion flux is proportional to the negative gradient of concentrations. It goes from regions of higher concentration to regions of lower concentration. Sometime later, various generalizations of Fick's laws were developed in the frame of thermodynamics and
The concept of diffusion is widely used in: physics ( particle diffusion), chemistry, biology, sociology, economics, and finance (diffusion of people, ideas and of price values). However, in each case the substance or collection undergoing diffusion is "spreading out" from a point or location at which there is a higher concentration of that substance or collection.
There are two ways to introduce the notion of ''diffusion'': either a phenomenological approach starting with Fick's laws of diffusion and their mathematical consequences, or a physical and atomistic one, by considering the '' random walk of the diffusing particles''.
In the phenomenological approach, ''diffusion is the movement of a substance from a region of high concentration to a region of low concentration without bulk motion''. According to Fick's laws, the diffusion flux is proportional to the negative gradient of concentrations. It goes from regions of higher concentration to regions of lower concentration. Sometime later, various generalizations of Fick's laws were developed in the frame of thermodynamics and non-equilibrium thermodynamics
Non-equilibrium thermodynamics is a branch of thermodynamics that deals with physical systems that are not in thermodynamic equilibrium but can be described in terms of macroscopic quantities (non-equilibrium state variables) that represent an ext ...
.
From the ''atomistic point of view'', diffusion is considered as a result of the random walk of the diffusing particles. In molecular diffusion
Molecular diffusion, often simply called diffusion, is the thermal motion of all (liquid or gas) particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size (mass) of ...
, the moving molecules are self-propelled by thermal energy. Random walk of small particles in suspension in a fluid was discovered in 1827 by Robert Brown, who found that minute particle suspended in a liquid medium and just large enough to be visible under an optical microscope exhibit a rapid and continually irregular motion of particles known as Brownian movement. The theory of the Brownian motion
Brownian motion, or pedesis (from grc, πήδησις "leaping"), is the random motion of particles suspended in a medium (a liquid or a gas).
This pattern of motion typically consists of random fluctuations in a particle's position insi ...
and the atomistic backgrounds of diffusion were developed by Albert Einstein.
The concept of diffusion is typically applied to any subject matter involving random walks in ensembles of individuals.
In chemistry and materials science, diffusion refers to the movement of fluid molecules in porous solids. Molecular diffusion
Molecular diffusion, often simply called diffusion, is the thermal motion of all (liquid or gas) particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size (mass) of ...
occurs when the collision with another molecule is more likely than the collision with the pore walls. Under such conditions, the diffusivity is similar to that in a non-confined space and is proportional to the mean free path. Knudsen diffusion
In physics, Knudsen diffusion, named after Martin Knudsen, is a means of diffusion that occurs when the scale length of a system is comparable to or smaller than the mean free path of the particles involved. An example of this is in a long pore w ...
, which occurs when the pore diameter is comparable to or smaller than the mean free path of the molecule diffusing through the pore. Under this condition, the collision with the pore walls becomes gradually more likely and the diffusivity is lower. Finally there is configurational diffusion, which happens if the molecules have comparable size to that of the pore. Under this condition, the diffusivity is much lower compared to molecular diffusion and small differences in the kinetic diameter of the molecule cause large differences in diffusivity.
Biologists often use the terms "net movement" or "net diffusion" to describe the movement of ions or molecules by diffusion. For example, oxygen can diffuse through cell membranes so long as there is a higher concentration of oxygen outside the cell. However, because the movement of molecules is random, occasionally oxygen molecules move out of the cell (against the concentration gradient). Because there are more oxygen molecules outside the cell, the probability that oxygen molecules will enter the cell is higher than the probability that oxygen molecules will leave the cell. Therefore, the "net" movement of oxygen molecules (the difference between the number of molecules either entering or leaving the cell) is into the cell. In other words, there is a ''net movement'' of oxygen molecules down the concentration gradient.
History of diffusion in physics
In the scope of time, diffusion in solids was used long before the theory of diffusion was created. For example,Pliny the Elder
Gaius Plinius Secundus (AD 23/2479), called Pliny the Elder (), was a Roman author, naturalist and natural philosopher, and naval and army commander of the early Roman Empire, and a friend of the emperor Vespasian. He wrote the encyclopedic ' ...
had previously described the cementation process, which produces steel from the element iron
Iron () is a chemical element with Symbol (chemistry), symbol Fe (from la, Wikt:ferrum, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 element, group 8 of the periodic table. It is, Abundanc ...
(Fe) through carbon diffusion. Another example is well known for many centuries, the diffusion of colors of stained glass or earthenware and Chinese ceramics.
In modern science, the first systematic experimental study of diffusion was performed by Thomas Graham. He studied diffusion in gases, and the main phenomenon was described by him in 1831–1833:
"...gases of different nature, when brought into contact, do not arrange themselves according to their density, the heaviest undermost, and the lighter uppermost, but they spontaneously diffuse, mutually and equally, through each other, and so remain in the intimate state of mixture for any length of time."The measurements of Graham contributed to James Clerk Maxwell deriving, in 1867, the coefficient of diffusion for CO2 in the air. The error rate is less than 5%. In 1855, Adolf Fick, the 26-year-old anatomy demonstrator from Zürich, proposed his law of diffusion. He used Graham's research, stating his goal as "the development of a fundamental law, for the operation of diffusion in a single element of space". He asserted a deep analogy between diffusion and conduction of heat or electricity, creating a formalism similar to Fourier's law for heat conduction (1822) and Ohm's law for electric current (1827). Robert Boyle demonstrated diffusion in solids in the 17th century by penetration of zinc into a copper coin. Nevertheless, diffusion in solids was not systematically studied until the second part of the 19th century. William Chandler Roberts-Austen, the well-known British metallurgist and former assistant of Thomas Graham studied systematically solid state diffusion on the example of gold in lead in 1896. :
"... My long connection with Graham's researches made it almost a duty to attempt to extend his work on liquid diffusion to metals."In 1858, Rudolf Clausius introduced the concept of the mean free path. In the same year, James Clerk Maxwell developed the first atomistic theory of transport processes in gases. The modern atomistic theory of diffusion and
Brownian motion
Brownian motion, or pedesis (from grc, πήδησις "leaping"), is the random motion of particles suspended in a medium (a liquid or a gas).
This pattern of motion typically consists of random fluctuations in a particle's position insi ...
was developed by Albert Einstein, Marian Smoluchowski and Jean-Baptiste Perrin. Ludwig Boltzmann
Ludwig Eduard Boltzmann (; 20 February 1844 – 5 September 1906) was an Austrian physicist and philosopher. His greatest achievements were the development of statistical mechanics, and the statistical explanation of the second law of ther ...
, in the development of the atomistic backgrounds of the macroscopic transport processes, introduced the Boltzmann equation
The Boltzmann equation or Boltzmann transport equation (BTE) describes the statistical behaviour of a thermodynamic system not in a state of equilibrium, devised by Ludwig Boltzmann in 1872.Encyclopaedia of Physics (2nd Edition), R. G. Lerne ...
, which has served mathematics and physics with a source of transport process ideas and concerns for more than 140 years.S. Chapman, T. G. Cowling (1970) ''The Mathematical Theory of Non-uniform Gases: An Account of the Kinetic Theory of Viscosity, Thermal Conduction and Diffusion in Gases'', Cambridge University Press (3rd edition), .
In 1920–1921, George de Hevesy
George Charles de Hevesy (born György Bischitz; hu, Hevesy György Károly; german: Georg Karl von Hevesy; 1 August 1885 – 5 July 1966) was a Hungarian radiochemist and Nobel Prize in Chemistry laureate, recognized in 1943 for his key rol ...
measured self-diffusion using radioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess nuclear energy, making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferr ...
s. He studied self-diffusion of radioactive isotopes of lead in the liquid and solid lead.
Yakov Frenkel
__NOTOC__
Yakov Il'ich Frenkel (russian: Яков Ильич Френкель; 10 February 1894 – 23 January 1952) was a Soviet physicist renowned for his works in the field of condensed matter physics. He is also known as Jacov Frenkel, frequ ...
(sometimes, Jakov/Jacob Frenkel) proposed, and elaborated in 1926, the idea of diffusion in crystals through local defects (vacancies and interstitial
An interstitial space or interstice is a space between structures or objects.
In particular, interstitial may refer to:
Biology
* Interstitial cell tumor
* Interstitial cell, any cell that lies between other cells
* Interstitial collagenase ...
atoms). He concluded, the diffusion process in condensed matter is an ensemble of elementary jumps and quasichemical interactions of particles and defects. He introduced several mechanisms of diffusion and found rate constants from experimental data.
Sometime later, Carl Wagner and Walter H. Schottky developed Frenkel's ideas about mechanisms of diffusion further. Presently, it is universally recognized that atomic defects are necessary to mediate diffusion in crystals.
Henry Eyring, with co-authors, applied his theory of absolute reaction rates to Frenkel's quasichemical model of diffusion. The analogy between reaction kinetics and diffusion leads to various nonlinear versions of Fick's law.
Basic models of diffusion
Diffusion flux
Each model of diffusion expresses the diffusion flux with the use of concentrations, densities and their derivatives. Flux is a vector representing the quantity and direction of transfer. Given a small area with normal , the transfer of a physical quantity through the area per time is : where is the inner product and is the little-o notation. If we use the notation ofvector area In 3-dimensional geometry and vector calculus, an area vector is a vector combining an area quantity with a direction, thus representing an ''oriented area'' in three dimensions.
Every bounded surface in three dimensions can be associated with ...
then
:
The dimension of the diffusion flux is luxnbsp;= uantity( ime� rea. The diffusing physical quantity may be the number of particles, mass, energy, electric charge, or any other scalar extensive quantity. For its density, , the diffusion equation has the form
:
where is intensity of any local source of this quantity (for example, the rate of a chemical reaction).
For the diffusion equation, the no-flux boundary conditions can be formulated as on the boundary, where is the normal to the boundary at point .
Fick's law and equations
Fick's first law: the diffusion flux is proportional to the negative of the concentration gradient: : The corresponding diffusion equation (Fick's second law) is : where is the Laplace operator, :Onsager's equations for multicomponent diffusion and thermodiffusion
Fick's law describes diffusion of an admixture in a medium. The concentration of this admixture should be small and the gradient of this concentration should be also small. The driving force of diffusion in Fick's law is the antigradient of concentration, . In 1931,Lars Onsager
Lars Onsager (November 27, 1903 – October 5, 1976) was a Norwegian-born American physical chemist and theoretical physicist. He held the Gibbs Professorship of Theoretical Chemistry at Yale University. He was awarded the Nobel Prize in C ...
included the multicomponent transport processes in the general context of linear non-equilibrium thermodynamics. For
multi-component transport,
:
where is the flux of the ''i''th physical quantity (component) and is the ''j''th thermodynamic force.
The thermodynamic forces for the transport processes were introduced by Onsager as the space gradients of the derivatives of the entropy density (he used the term "force" in quotation marks or "driving force"):
:
where are the "thermodynamic coordinates".
For the heat and mass transfer one can take (the density of internal energy) and is the concentration of the th component. The corresponding driving forces are the space vectors
: because
where ''T'' is the absolute temperature and is the chemical potential of the th component. It should be stressed that the separate diffusion equations describe the mixing or mass transport without bulk motion. Therefore, the terms with variation of the total pressure are neglected. It is possible for diffusion of small admixtures and for small gradients.
For the linear Onsager equations, we must take the thermodynamic forces in the linear approximation near equilibrium:
:
where the derivatives of are calculated at equilibrium .
The matrix of the ''kinetic coefficients'' should be symmetric (Onsager reciprocal relations
In thermodynamics, the Onsager reciprocal relations express the equality of certain ratios between flows and forces in thermodynamic systems out of equilibrium, but where a notion of local equilibrium exists.
"Reciprocal relations" occur betwe ...
) and positive definite ( for the entropy growth).
The transport equations are
:
Here, all the indexes are related to the internal energy (0) and various components. The expression in the square brackets is the matrix of the diffusion (''i'',''k'' > 0), thermodiffusion (''i'' > 0, ''k'' = 0 or ''k'' > 0, ''i'' = 0) and thermal conductivity () coefficients.
Under isothermal conditions ''T'' = constant. The relevant thermodynamic potential is the free energy (or the free entropy
A thermodynamic free entropy is an entropic thermodynamic potential analogous to the free energy. Also known as a Massieu, Planck, or Massieu–Planck potentials (or functions), or (rarely) free information. In statistical mechanics, free entropi ...
). The thermodynamic driving forces for the isothermal diffusion are antigradients of chemical potentials, , and the matrix of diffusion coefficients is
:
(''i,k'' > 0).
There is intrinsic arbitrariness in the definition of the thermodynamic forces and kinetic coefficients because they are not measurable separately and only their combinations can be measured. For example, in the original work of Onsager the thermodynamic forces include additional multiplier ''T'', whereas in the Course of Theoretical Physics
The ''Course of Theoretical Physics'' is a ten-volume series of books covering theoretical physics that was initiated by Lev Landau and written in collaboration with his student Evgeny Lifshitz starting in the late 1930s.
It is said that Land ...
this multiplier is omitted but the sign of the thermodynamic forces is opposite. All these changes are supplemented by the corresponding changes in the coefficients and do not affect the measurable quantities.
Nondiagonal diffusion must be nonlinear
The formalism of linear irreversible thermodynamics (Onsager) generates the systems of linear diffusion equations in the form : If the matrix of diffusion coefficients is diagonal, then this system of equations is just a collection of decoupled Fick's equations for various components. Assume that diffusion is non-diagonal, for example, , and consider the state with . At this state, . If at some points, then becomes negative at these points in a short time. Therefore, linear non-diagonal diffusion does not preserve positivity of concentrations. Non-diagonal equations of multicomponent diffusion must be non-linear.Einstein's mobility and Teorell formula
The Einstein relation (kinetic theory) connects the diffusion coefficient and the mobility (the ratio of the particle's terminal drift velocity to an applied force) : where ''D'' is the diffusion constant, ''μ'' is the "mobility", ''k''B isBoltzmann's constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin and the gas constant ...
, ''T'' is the absolute temperature, and ''q'' is the elementary charge, that is, the charge of one electron.
Below, to combine in the same formula the chemical potential ''μ'' and the mobility, we use for mobility the notation .
The mobility-based approach was further applied by T. Teorell. In 1935, he studied the diffusion of ions through a membrane. He formulated the essence of his approach in the formula:
:the flux is equal to mobility × concentration × force per gram-ion.
This is the so-called ''Teorell formula''. The term "gram-ion" ("gram-particle") is used for a quantity of a substance that contains Avogadro's number of ions (particles). The common modern term is mole
Mole (or Molé) may refer to:
Animals
* Mole (animal) or "true mole", mammals in the family Talpidae, found in Eurasia and North America
* Golden moles, southern African mammals in the family Chrysochloridae, similar to but unrelated to Talpida ...
.
The force under isothermal conditions consists of two parts:
# Diffusion force caused by concentration gradient: .
# Electrostatic force caused by electric potential gradient: .
Here ''R'' is the gas constant, ''T'' is the absolute temperature, ''n'' is the concentration, the equilibrium concentration is marked by a superscript "eq", ''q'' is the charge and ''φ'' is the electric potential.
The simple but crucial difference between the Teorell formula and the Onsager laws is the concentration factor in the Teorell expression for the flux. In the Einstein–Teorell approach, if for the finite force the concentration tends to zero then the flux also tends to zero, whereas the Onsager equations violate this simple and physically obvious rule.
The general formulation of the Teorell formula for non-perfect systems under isothermal conditions is
:
where ''μ'' is the chemical potential, ''μ''0 is the standard value of the chemical potential.
The expression is the so-called activity. It measures the "effective concentration" of a species in a non-ideal mixture. In this notation, the Teorell formula for the flux has a very simple form
:
The standard derivation of the activity includes a normalization factor and for small concentrations , where is the standard concentration. Therefore, this formula for the flux describes the flux of the normalized dimensionless quantity :
:
Fluctuation-dissipation theorem
Fluctuation-dissipation theorem
The fluctuation–dissipation theorem (FDT) or fluctuation–dissipation relation (FDR) is a powerful tool in statistical physics for predicting the behavior of systems that obey detailed balance. Given that a system obeys detailed balance, the th ...
based on the Langevin equation is developed to extend the Einstein model to the ballistic time scale. According to Langevin, the equation is based on Newton's second law of motion as
:
where
* ''x'' is the position.
* ''μ'' is the mobility of the particle in the fluid or gas, which can be calculated using the Einstein relation (kinetic theory).
* ''m'' is the mass of the particle.
* ''F'' is the random force applied to the particle.
* ''t'' is time.
Solving this equation, one obtained the time-dependent diffusion constant in the long-time limit and when the particle is significantly denser than the surrounding fluid,
:
where
* ''k''B is Boltzmann's constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative kinetic energy of particles in a gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin and the gas constant ...
;
* ''T'' is the absolute temperature.
* ''μ'' is the mobility of the particle in the fluid or gas, which can be calculated using the Einstein relation (kinetic theory).
* ''m'' is the mass of the particle.
* ''t'' is time.
Teorell formula for multicomponent diffusion
The Teorell formula with combination of Onsager's definition of the diffusion force gives : where is the mobility of the ''i''th component, is its activity, is the matrix of the coefficients, is the thermodynamic diffusion force, . For the isothermal perfect systems, . Therefore, the Einstein–Teorell approach gives the following multicomponent generalization of the Fick's law for multicomponent diffusion: : where is the matrix of coefficients. The Chapman–Enskog formulas for diffusion in gases include exactly the same terms. Earlier, such terms were introduced in theMaxwell–Stefan diffusion
The Maxwell–Stefan diffusion (or Stefan–Maxwell diffusion) is a model for describing diffusion in multicomponent systems. The equations that describe these transport processes have been developed independently and in parallel by James Clerk Max ...
equation.
Jumps on the surface and in solids
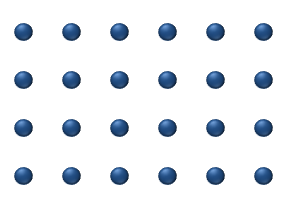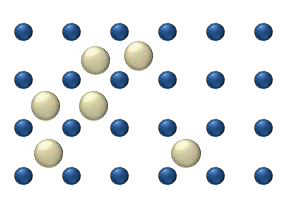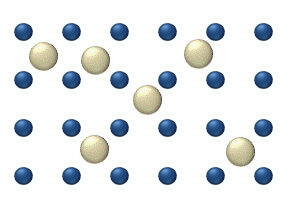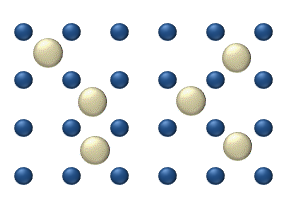 Diffusion of reagents on the surface of a catalyst may play an important role in heterogeneous catalysis. The model of diffusion in the ideal monolayer is based on the jumps of the reagents on the nearest free places. This model was used for CO on Pt oxidation under low gas pressure.
The system includes several reagents on the surface. Their surface concentrations are The surface is a lattice of the adsorption places. Each
reagent molecule fills a place on the surface. Some of the places are free. The concentration of the free places is . The sum of all (including free places) is constant, the density of adsorption places ''b''.
The jump model gives for the diffusion flux of (''i'' = 1, ..., ''n''):
:
The corresponding diffusion equation is:
:
Due to the conservation law, and we
have the system of ''m'' diffusion equations. For one component we get Fick's law and linear equations because . For two and more components the equations are nonlinear.
If all particles can exchange their positions with their closest neighbours then a simple generalization gives
:
Diffusion of reagents on the surface of a catalyst may play an important role in heterogeneous catalysis. The model of diffusion in the ideal monolayer is based on the jumps of the reagents on the nearest free places. This model was used for CO on Pt oxidation under low gas pressure.
The system includes several reagents on the surface. Their surface concentrations are The surface is a lattice of the adsorption places. Each
reagent molecule fills a place on the surface. Some of the places are free. The concentration of the free places is . The sum of all (including free places) is constant, the density of adsorption places ''b''.
The jump model gives for the diffusion flux of (''i'' = 1, ..., ''n''):
:
The corresponding diffusion equation is:
:
Due to the conservation law, and we
have the system of ''m'' diffusion equations. For one component we get Fick's law and linear equations because . For two and more components the equations are nonlinear.
If all particles can exchange their positions with their closest neighbours then a simple generalization gives
: