Myoglobin on:
[Wikipedia]
[Google]
[Amazon]
Myoglobin (symbol Mb or MB) is an
/ref> Despite being one of the most studied proteins in biology, its physiological function is not yet conclusively established: mice genetically engineered to lack myoglobin can be viable and fertile, but show many cellular and physiological adaptations to overcome the loss. Through observing these changes in myoglobin-depleted mice, it is hypothesised that myoglobin function relates to increased oxygen transport to muscle, and to oxygen storage; as well, it serves as a scavenger of reactive oxygen species. In humans, myoglobin is encoded by the ''MB''
File:MbO2MO.png, Molecular orbital description of Fe-O2 interaction in myoglobin.
File:1a6m Oxy-Myoglobin.jpg, This is an image of an oxygenated myoglobin molecule. The image shows the structural change when oxygen is bound to the iron atom of the heme prosthetic group. The oxygen atoms are colored in green, the iron atom is colored in red, and the heme group is colored in blue.
Proteopedia article about this finding
The Myoglobin Protein
''The New York Times'', February 21, 2006 article regarding meat industry use of carbon monoxide to keep meat looking red.
''The New York Times'', March 1, 2006 article on the use of carbon monoxide to make meat appear fresh. * {{Authority control Hemoproteins Human proteins
iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
- and oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
-binding protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
found in the cardiac and skeletal
A skeleton is the structural frame that supports the body of an animal. There are several types of skeletons, including the exoskeleton, which is the stable outer shell of an organism, the endoskeleton, which forms the support structure inside ...
muscle tissue of vertebrates in general and in almost all mammals. Myoglobin is distantly related to hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythroc ...
. Compared to hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythroc ...
, myoglobin has a higher affinity for oxygen and does not have cooperative binding
Molecular binding is an interaction between molecules that results in a stable physical association between those molecules. Cooperative binding occurs in binding systems containing more than one type, or species, of molecule and in which one of t ...
with oxygen like hemoglobin does. In humans, myoglobin is only found in the bloodstream after muscle injury. (Google books link is the 2008 edition)
High concentrations of myoglobin in muscle cells allow organisms to hold their breath for a longer period of time. Diving mammals such as whales and seals have muscles with particularly high abundance of myoglobin. Myoglobin is found in Type I muscle, Type II A, and Type II B; although many texts consider myoglobin not to be found in smooth muscle
Smooth muscle is an involuntary non- striated muscle, so-called because it has no sarcomeres and therefore no striations (''bands'' or ''stripes''). It is divided into two subgroups, single-unit and multiunit smooth muscle. Within single-unit ...
, this has proved erroneous: there is also myoglobin in smooth muscle cells.
Myoglobin was the first protein to have its three-dimensional structure revealed by X-ray crystallography. This achievement was reported in 1958 by John Kendrew
Sir John Cowdery Kendrew, (24 March 1917 – 23 August 1997) was an English biochemist, crystallographer, and science administrator. Kendrew shared the 1962 Nobel Prize in Chemistry with Max Perutz, for their work at the Cavendish Lab ...
and associates. For this discovery, Kendrew shared the 1962 Nobel Prize in chemistry
)
, image = Nobel Prize.png
, alt = A golden medallion with an embossed image of a bearded man facing left in profile. To the left of the man is the text "ALFR•" then "NOBEL", and on the right, the text (smaller) "NAT•" then "M ...
with Max Perutz
Max Ferdinand Perutz (19 May 1914 – 6 February 2002) was an Austrian-born British molecular biologist, who shared the 1962 Nobel Prize for Chemistry with John Kendrew, for their studies of the structures of haemoglobin and myoglobin. He went ...
.The Nobel Prize in Chemistry 1962/ref> Despite being one of the most studied proteins in biology, its physiological function is not yet conclusively established: mice genetically engineered to lack myoglobin can be viable and fertile, but show many cellular and physiological adaptations to overcome the loss. Through observing these changes in myoglobin-depleted mice, it is hypothesised that myoglobin function relates to increased oxygen transport to muscle, and to oxygen storage; as well, it serves as a scavenger of reactive oxygen species. In humans, myoglobin is encoded by the ''MB''
gene
In biology, the word gene (from , ; "...Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a b ...
.
Myoglobin can take the forms oxymyoglobin (MbO2), carboxymyoglobin (MbCO), and metmyoglobin (met-Mb), analogously to hemoglobin taking the forms oxyhemoglobin (HbO2), carboxyhemoglobin (HbCO), and methemoglobin (met-Hb).
Differences from hemoglobin
Like hemoglobin, myoglobin is a cytoplasmic protein that binds oxygen on a heme group. It harbors only one globulin group, whereas hemoglobin has four. Although its heme group is identical to those in Hb, Mb has a higher affinity for oxygen than does hemoglobin. This difference is related to its different role: whereas hemoglobin transports oxygen, myoglobin's function is to store oxygen.Role in cuisine
Myoglobin contains hemes,pigment
A pigment is a colored material that is completely or nearly insoluble in water. In contrast, dyes are typically soluble, at least at some stage in their use. Generally dyes are often organic compounds whereas pigments are often inorganic compou ...
s responsible for the colour of red meat
In gastronomy, red meat is commonly red when raw and a dark color after it is cooked, in contrast to white meat, which is pale in color before and after cooking. In culinary terms, only flesh from mammals or fowl (not fish) is classified a ...
. The colour that meat takes is partly determined by the degree of oxidation of the myoglobin. In fresh meat the iron atom is in the ferrous (+2) oxidation state bound to an oxygen molecule (O2). Meat cooked well done
Doneness is a gauge of how thoroughly cooked a cut of meat is based on its color, juiciness, and internal temperature. The gradations are most often used in reference to beef (especially steaks and roasts) but are also applicable to other type ...
is brown because the iron atom is now in the ferric (+3) oxidation state, having lost an electron. If meat has been exposed to nitrites, it will remain pink because the iron atom is bound to NO, nitric oxide
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its ...
(true of, e.g., corned beef or cured hams). Grilled meats can also take on a reddish pink "smoke ring" that comes from the heme center binding to carbon monoxide
Carbon monoxide ( chemical formula CO) is a colorless, poisonous, odorless, tasteless, flammable gas that is slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simpl ...
. Raw meat packed in a carbon monoxide atmosphere also shows this same pink "smoke ring" due to the same principles. Notably, the surface of this raw meat also displays the pink color, which is usually associated in consumers' minds with fresh meat. This artificially induced pink color can persist, reportedly up to one year. Hormel and Cargill (meat processing companies in the US) are both reported to use this meat-packing process, and meat treated this way has been in the consumer market since 2003.
Role in disease
Myoglobin is released from damaged muscle tissue ( rhabdomyolysis), which has very high concentrations of myoglobin. The released myoglobin is filtered by the kidneys but is toxic to the renal tubular epithelium and so may cause acute kidney injury. It is not the myoglobin itself that is toxic (it is a protoxin) but the ferrihemate portion that is dissociated from myoglobin in acidic environments (e.g., acidic urine,lysosome
A lysosome () is a membrane-bound organelle found in many animal cells. They are spherical vesicles that contain hydrolytic enzymes that can break down many kinds of biomolecules. A lysosome has a specific composition, of both its membrane p ...
s).
Myoglobin is a sensitive marker for muscle injury, making it a potential marker for heart attack
A myocardial infarction (MI), commonly known as a heart attack, occurs when blood flow decreases or stops to the coronary artery of the heart, causing damage to the heart muscle. The most common symptom is chest pain or discomfort which ma ...
in patients with chest pain. However, elevated myoglobin has low specificity for acute myocardial infarction (AMI) and thus CK-MB, cardiac troponin, ECG, and clinical signs should be taken into account to make the diagnosis.
Structure and bonding
Myoglobin belongs to the globin superfamily of proteins, and as with other globins, consists of eight alpha helices connected by loops. Myoglobin contains 153 amino acids. Myoglobin contains a porphyrin ring with an iron at its center. A ''proximal''histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the ...
group (His-93) is attached directly to iron, and a ''distal'' histidine group (His-64) hovers near the opposite face. The distal imidazole is not bonded to the iron but is available to interact with the substrate O2. This interaction encourages the binding of O2, but not carbon monoxide (CO), which still binds about 240× more strongly than O2.
The binding of O2 causes substantial structural change at the Fe center, which shrinks in radius and moves into the center of N4 pocket. O2-binding induces "spin-pairing": the five-coordinate ferrous deoxy form is high spin Spin states when describing transition metal coordination complexes refers to the potential spin configurations of the central metal's d electrons. For several oxidation states, metals can adopt high-spin and low-spin configurations. The ambiguity o ...
and the six coordinate oxy form is low spin and diamagnetic.
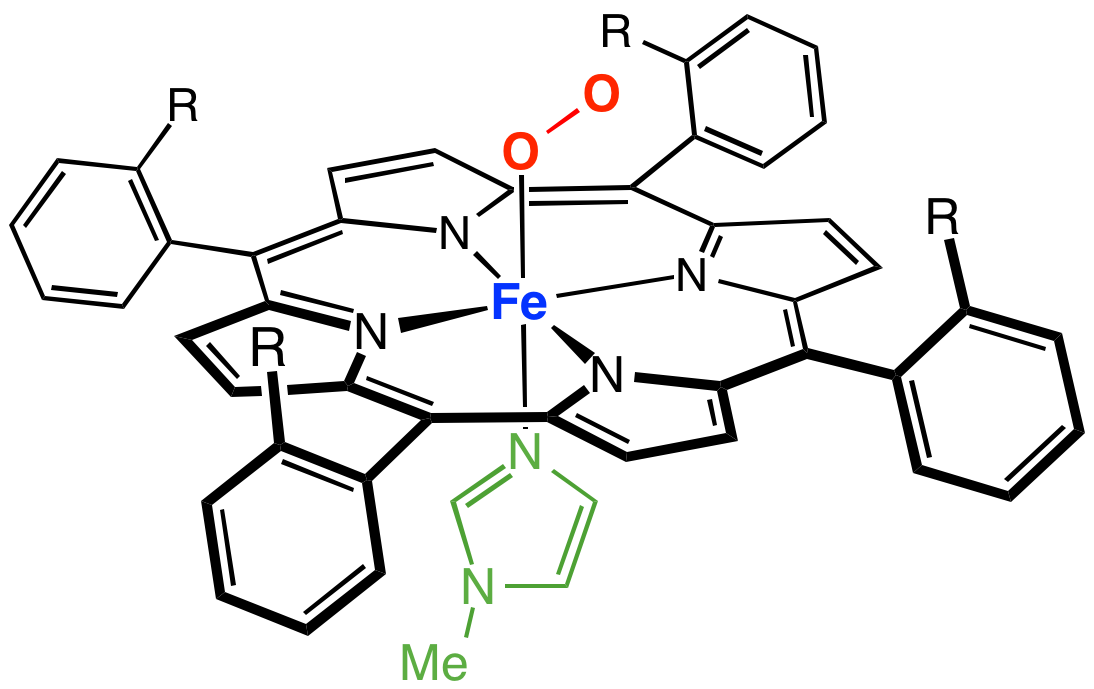
Synthetic analogues
Many models of myoglobin have been synthesized as part of a broad interest intransition metal dioxygen complex
Transition or transitional may refer to:
Mathematics, science, and technology Biology
* Transition (genetics), a point mutation that changes a purine nucleotide to another purine (A ↔ G) or a pyrimidine nucleotide to another pyrimidine (C ↔ ...
es. A well known example is the ''picket fence porphyrin'', which consists of a ferrous complex of a sterically bulky derivative of tetraphenylporphyrin. In the presence of an imidazole
Imidazole (ImH) is an organic compound with the formula C3N2H4. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. In chemistry, it is an aromatic heterocycle, classified as a diazole, and has non ...
ligand, this ferrous complex reversibly binds O2. The O2 substrate adopts a bent geometry, occupying the sixth position of the iron center. A key property of this model is the slow formation of the μ-oxo dimer, which is an inactive diferric state. In nature, such deactivation pathways are suppressed by protein matrix that prevents close approach of the Fe-porphyrin assemblies.
:
See also
* Cytoglobin *Hemoglobin
Hemoglobin (haemoglobin BrE) (from the Greek word αἷμα, ''haîma'' 'blood' + Latin ''globus'' 'ball, sphere' + ''-in'') (), abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein present in red blood cells (erythroc ...
* Hemoprotein
* Neuroglobin
* Phytoglobin
Phytoglobins are globular plant (algae and land plant) proteins classified into the globin superfamily, which contain a heme, ''i''.''e''. protoporphyrin IX-Fe, prosthetic group. The earliest known phytoglobins are leghemoglobins, discovered in ...
* Myoglobinuria
Myoglobinuria is the presence of myoglobin in the urine, which usually results from rhabdomyolysis or muscle injury. Myoglobin is present in muscle cells as a reserve of oxygen.
Signs and symptoms
Signs and symptoms of myoglobinuria are us ...
- The presence of myoglobin in the urine
* Ischemia-reperfusion injury of the appendicular musculoskeletal system Ischemia-reperfusion (IR) tissue injury is the resultant pathology from a combination of factors, including tissue hypoxia, followed by tissue damage associated with re-oxygenation. IR injury contributes to disease and mortality in a variety of pa ...
References
Further reading
* * * * * * * * . Also seProteopedia article about this finding
External links
* human geneticsThe Myoglobin Protein
''The New York Times'', February 21, 2006 article regarding meat industry use of carbon monoxide to keep meat looking red.
''The New York Times'', March 1, 2006 article on the use of carbon monoxide to make meat appear fresh. * {{Authority control Hemoproteins Human proteins