aconitase on:
[Wikipedia]
[Google]
[Amazon]
Aconitase (aconitate hydratase; ) is an enzyme that catalyses the stereo-specific
Image:Citrate wpmp.png,
Image:Cis-Aconitate wpmp.png,
Image:isocitric acid.svg,
isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomeri ...
of citrate
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in t ...
to isocitrate
Isocitric acid is a structural isomer of citric acid. Since citric acid and isocitric acid are structural isomers, they share similar physical and chemical properties. Due to these similar properties, it is difficult to separate the isomers. Salts ...
via ''cis''-aconitate
Aconitic acid is an organic acid. The two isomers are ''cis''-aconitic acid and ''trans''-aconitic acid. The conjugate base of ''cis''-aconitic acid, ''cis''-aconitate is an intermediate in the isomerization of citrate to isocitrate in the citric ...
in the tricarboxylic acid cycle
The citric acid cycle (CAC)—also known as the Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins ...
, a non- redox-active process.
Structure
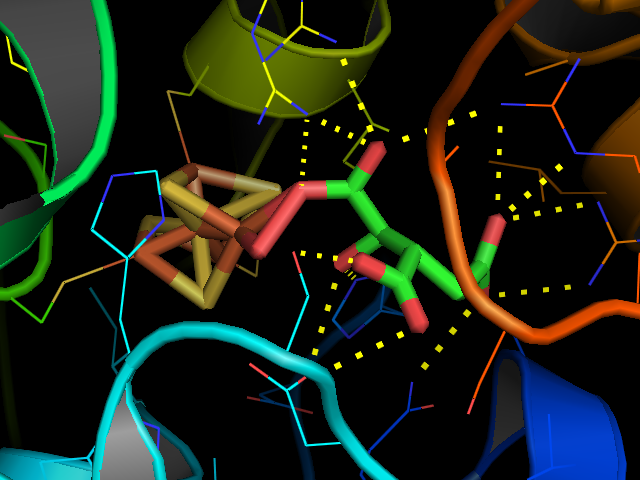
Aconitase, displayed in the structures in the right margin of this page, has two slightly different structures, depending on whether it is activated or inactivated. In the inactive form, its structure is divided into four domains. Counting from the N-terminus, only the first three of these domains are involved in close interactions with the Fe-4Scluster, but theactive site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate (binding site) ...
consists of residues from all four domains, including the larger C-terminal
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain ( protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is ...
domain. The Fe-S cluster and a anion also reside in the active site. When the enzyme is activated, it gains an additional iron atom, creating a Fe-4Scluster. However, the structure of the rest of the enzyme is nearly unchanged; the conserved atoms between the two forms are in essentially the same positions, up to a difference of 0.1 angstroms.
Function
In contrast with the majority ofiron-sulfur protein
Iron–sulfur proteins (or iron–sulphur proteins in British spelling) are proteins characterized by the presence of iron–sulfur clusters containing sulfide-linked di-, tri-, and tetrairon centers in variable oxidation states. Iron–sulfur ...
s that function as electron carriers, the iron-sulfur cluster of aconitase reacts directly with an enzyme substrate. Aconitase has an active e4S4sup>2+ cluster, which may convert to an inactive e3S4sup>+ form. Three cysteine (Cys) residues have been shown to be ligands of the e4S4centre. In the active state, the labile iron ion of the e4S4cluster is not coordinated by Cys but by water molecules.
The iron-responsive element-binding protein
The iron-responsive element-binding proteins, also known as IRE-BP, IRBP, IRP and IFR
, bind to iron-responsive elements (IREs) in the regulation of human iron metabolism.
Function
ACO1, or IRP1, is a bifunctional protein that functions as an ...
(IRE-BP) and 3-isopropylmalate dehydratase
3-Isopropylmalate dehydratase () is an aconitase homologue, which catalyses the isomerisation of 2-isopropylmalate to 3-isopropylmalate, via dehydration, in the biosynthesis of leucine
Leucine (symbol Leu or L) is an essential amino acid that i ...
(α-isopropylmalate isomerase; ), an enzyme catalysing the second step in the biosynthesis of leucine
Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α- c ...
, are known aconitase homologues. Iron regulatory elements (IREs) constitute a family of 28-nucleotide, non-coding, stem-loop structures that regulate iron storage, heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver.
In biochemical terms, heme is a coordination complex "consist ...
synthesis and iron uptake. They also participate in ribosome binding and control the mRNA turnover (degradation). The specific regulator protein, the IRE-BP, binds to IREs in both 5' and 3' regions, but only to RNA in the apo form, without the Fe-S cluster. Expression of IRE-BP in cultured cells has revealed that the protein functions either as an active aconitase, when cells are iron-replete, or as an active RNA-binding protein, when cells are iron-depleted. Mutant IRE-BPs, in which any or all of the three Cys residues involved in Fe-S formation are replaced by serine, have no aconitase activity, but retain RNA-binding properties.
Aconitase is inhibited by fluoroacetate
Fluoroacetate may refer to:
* Fluoroacetic acid
* Sodium fluoroacetate
Sodium fluoroacetate is an organofluorine chemical compound with the formula FCH2CO2Na. This colourless salt has a taste similar to that of sodium chloride and is used as a ...
, therefore fluoroacetate is poisonous. Fluoroacetate, in the citric acid cycle, can innocently enter as fluorocitrate. However, aconitase cannot bind this substrate and thus the citric acid cycle is halted. The iron sulfur cluster is highly sensitive to oxidation by superoxide
In chemistry, a superoxide is a compound that contains the superoxide ion, which has the chemical formula . The systematic name of the anion is dioxide(1−). The reactive oxygen ion superoxide is particularly important as the product of th ...
.
Mechanism
Aconitase employs a dehydration-hydration mechanism. The catalytic residues involved are His-101 and Ser-642. His-101 protonates the hydroxyl group on C3 of citrate, allowing it to leave as water, and Ser-642 concurrently abstracts the proton on C2, creating a double bond between C2 and C3, and forming the so-called ''cis''-aconitate intermediate (the twocarboxyl group
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
s on the double bond are ''cis''). The carbon atom from which the hydrogen is removed is the one that came from oxaloacetate in the previous step of the citric acid cycle, not the one that came from acetyl CoA
Acetyl-CoA (acetyl coenzyme A) is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle (Krebs cycle) to be oxidized fo ...
, even though these two carbons are equivalent except that one is "''pro''-R" and the other "''pro''-S" (see Prochirality
In stereochemistry, prochiral molecules are those that can be converted from achiral to chiral in a single step. An achiral species which can be converted to a chiral in two steps is called proprochiral.
If two identical substituents are attach ...
). At this point, the intermediate is rotated 180°. This rotation is referred to as a "flip." Because of this flip, the intermediate is said to move from a "citrate mode" to a "isocitrate mode."
How exactly this flip occurs is debatable. One theory is that, in the rate-limiting step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the ...
of the mechanism, the ''cis''-aconitate is released from the enzyme, then reattached in the isocitrate mode to complete the reaction. This rate-limiting step ensures that the right stereochemistry, specifically (2R,3S), is formed in the final product. Another hypothesis is that ''cis''-aconitate stays bound to the enzyme while it flips from the citrate to the isocitrate mode.
In either case, flipping ''cis''-aconitate allows the dehydration and hydration steps to occur on opposite faces of the intermediate. Aconitase catalyzes ''trans'' elimination/addition of water, and the flip guarantees that the correct stereochemistry is formed in the product. To complete the reaction, the serine and histidine residues reverse their original catalytic actions: the histidine, now basic, abstracts a proton from water, priming it as a nucleophile to attack at C2, and the protonated serine is deprotonated by the ''cis''-aconitate double bond to complete the hydration, producing isocitrate.

Family members
Aconitases are expressed in bacteria to humans. Humans express the following two aconitase isozymes:Interactive pathway map
References
Further reading
*External links
* * - the Aconitase structure in interactive 3D {{Portal bar, Biology, border=no EC 4.2.1 Iron–sulfur proteins Moonlighting proteins