|
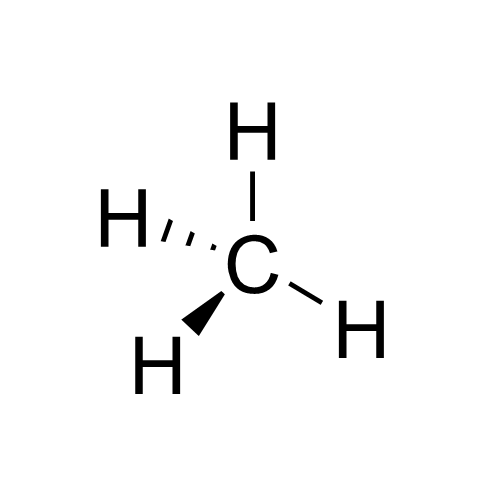
Prochirality
In stereochemistry, prochiral molecules are those that can be converted from achiral to chiral in a single step. An achiral species which can be converted to a chiral in two steps is called proprochiral. If two identical substituents are attached to a sp3-hybridized atom, the descriptors ''pro''-R and ''pro''-S are used to distinguish between the two. Promoting the ''pro''-R substituent to higher priority than the other identical substituent results in an ''R'' chirality center at the original sp3-hybridized atom, and analogously for the ''pro''-S substituent. A trigonal planar sp2-hybridized atom can be converted to a chiral center when a substituent is added to the ''re'' or ''si'' () face of the molecule. A face is labeled ''re'' if, when looking at that face, the substituents at the trigonal atom are arranged in increasing Cahn-Ingold-Prelog priority order (1 to 2 to 3) in a clockwise order, and ''si'' if the priorities increase in anti-clockwise order; note that the desi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alexander George Ogston
Alexander George Ogston FAA FRS (30 January 1911 – 29 June 1996) was a British biochemist who specialised in the thermodynamics of biological systems. He was a grandson of Sir Alexander Ogston, a Scottish surgeon who discovered ''Staphylococcus''. Life Ogston was educated at Eton College and Balliol College, Oxford. Apart from a period as Freedom Research Fellow at the London Hospital, he spent most of his career at Oxford, being appointed Demonstrator (1938) and Reader (1955) in Biochemistry, and Fellow and Tutor in Physical Chemistry at Balliol (1937). In that capacity he had a major influence on other distinguished scientists, such as the Nobel prizewinner Oliver Smithies, who wrote his first paper with him, and Richard Dawkins, who chose to study zoology on his recommendation. In 1959 he took up an appointment as Professor of Physical Biochemistry at the John Curtin School of Medical Research at the Australian National University (ANU), Canberra, where he remained until ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prochirality V
In stereochemistry, prochiral molecules are those that can be converted from achiral to chiral in a single step. An achiral species which can be converted to a chiral in two steps is called proprochiral. If two identical substituents are attached to a sp3-hybridized atom, the descriptors ''pro''-R and ''pro''-S are used to distinguish between the two. Promoting the ''pro''-R substituent to higher priority than the other identical substituent results in an ''R'' chirality center at the original sp3-hybridized atom, and analogously for the ''pro''-S substituent. A trigonal planar sp2-hybridized atom can be converted to a chiral center when a substituent is added to the ''re'' or ''si'' () face of the molecule. A face is labeled ''re'' if, when looking at that face, the substituents at the trigonal atom are arranged in increasing Cahn-Ingold-Prelog priority order (1 to 2 to 3) in a clockwise order, and ''si'' if the priorities increase in anti-clockwise order; note that the desig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which by definition have the same molecular formula and sequence of bonded atoms (constitution), but differ in structural formula (the three-dimensional orientations of their atoms in space). For this reason, it is also known as 3D chemistry—the prefix "stereo-" means "three-dimensionality". Stereochemistry spans the entire spectrum of organic, inorganic, biological, physical and especially supramolecular chemistry. Stereochemistry includes methods for determining and describing these relationships; the effect on the physical or biological properties these relationships impart upon the molecules in question, and the manner in which these relationships influence the reactivity of the molecules in question (dynamic stereochemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chirality (chemistry)
In chemistry, a molecule or ion is called chiral () if it cannot be superposed on its mirror image by any combination of rotations, translations, and some conformational changes. This geometric property is called chirality (). The terms are derived from Ancient Greek χείρ (''cheir'') 'hand'; which is the canonical example of an object with this property. A chiral molecule or ion exists in two stereoisomers that are mirror images of each other, called enantiomers; they are often distinguished as either "right-handed" or "left-handed" by their absolute configuration or some other criterion. The two enantiomers have the same chemical properties, except when reacting with other chiral compounds. They also have the same physical properties, except that they often have opposite optical activities. A homogeneous mixture of the two enantiomers in equal parts is said to be racemic, and it usually differs chemically and physically from the pure enantiomers. Chiral molecules ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Step
A reaction step of a chemical reaction is defined as: ''"An elementary reaction, constituting one of the stages of a stepwise reaction in which a reaction intermediate (or, for the first step, the reactants) is converted into the next reaction intermediate (or, for the last step, the products) in the sequence of intermediates between reactants and products"''. – Gold Book
The International Union of Pure and Applied Chemistry publishes many books which contain its complete list of definitions. The definitions are divided into seven "colour books": Gold, Green, Blue, Purple, ...
[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituent
A substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. (In organic chemistry and biochemistry, the terms ''substituent'' and ''functional group'', as well as '' side chain'' and ''pendant group'', are used almost interchangeably to describe those branches from the parent structure, though certain distinctions are made in polymer chemistry. In polymers, side chains extend from the backbone structure. In proteins, side chains are attached to the alpha carbon atoms of the amino acid backbone.) The suffix ''-yl'' is used when naming organic compounds that contain a single bond replacing one hydrogen; ''-ylidene'' and ''-ylidyne'' are used with double bonds and triple bonds, respectively. In addition, when naming hydrocarbons that contain a substituent, positional numbers are used to indicate which carbon atom the substituent attaches to when such information is needed to distinguish between isomers. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orbital Hybridisation
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory. For example, in a carbon atom which forms four single bonds the valence-shell s orbital combines with three valence-shell p orbitals to form four equivalent sp3 mixtures in a tetrahedral arrangement around the carbon to bond to four different atoms. Hybrid orbitals are useful in the explanation of molecular geometry and atomic bonding properties and are symmetrically disposed in space. Usually hybrid orbitals are formed by mixing atomic orbitals of comparable energies. History and uses Chemist Linus Pauling first developed the hybridisation theory in 1931 to explain the structure of simple molecules such as methane (CH4) using atomic orbitals. Pauling pointed out that a carbon atom forms fou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small, typically around 100 picometers across. They are so small that accurately predicting their behavior using classical physics, as if they were tennis balls for example, is not possible due to quantum effects. More than 99.94% of an atom's mass is in the nucleus. The protons have a positive electric charge, the electrons have a negative electric charge, and the neutrons have no electric charge. If the number of protons and electrons are equal, then the atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively – such atoms are called ions. The electrons of an atom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
VSEPR
Valence shell electron pair repulsion (VSEPR) theory ( , ), is a model used in chemistry to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms. It is also named the Gillespie-Nyholm theory after its two main developers, Ronald Gillespie and Ronald Nyholm. The premise of VSEPR is that the valence electron pairs surrounding an atom tend to repel each other and will, therefore, adopt an arrangement that minimizes this repulsion. This in turn decreases the molecule's energy and increases its stability, which determines the molecular geometry. Gillespie has emphasized that the electron-electron repulsion due to the Pauli exclusion principle is more important in determining molecular geometry than the electrostatic repulsion. The insights of VSEPR theory are derived from topological analysis of the electron density of molecules. Such quantum chemical topology (QCT) methods include the electron localization function (ELF) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereospecificity
In chemistry, stereospecificity is the property of a reaction mechanism that leads to different stereoisomeric reaction products from different stereoisomeric reactants, or which operates on only one (or a subset) of the stereoisomers."Overlap Control of Carbanionoid Reactions. I. Stereoselectivity in Alkaline Epoxidation," Zimmerman, H. E.; Singer, L.; Thyagarajan, B. S. J. Am. Chem. Soc., 1959, 81, 108-116.Eliel, E., "Stereochemistry of Carbon Compound", McGraw-Hill, 1962 pp 434-436 In contrast, stereoselectivity is the property of a reactant mixture where a non-stereospecific mechanism allows for the formation of multiple products, but where one (or a subset) of the products is favored by factors, such as steric access, that are independent of the mechanism. A stereospecific mechanism ''specifies'' the stereochemical outcome of a given reactant, whereas a stereoselective reaction ''selects'' products from those made available by the same, non-specific mechanism acting on a g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the react ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |