Zero Temperature on:
[Wikipedia]
[Google]
[Amazon]
 Absolute zero is the lowest limit of the thermodynamic temperature scale, a state at which the enthalpy and entropy of a cooled ideal gas reach their minimum value, taken as zero kelvin. The fundamental particles of nature have minimum vibrational motion, retaining only quantum mechanical, zero-point energy-induced particle motion. The theoretical temperature is determined by extrapolating the ideal gas law; by international agreement, absolute zero is taken as −273.15 degrees on the
Absolute zero is the lowest limit of the thermodynamic temperature scale, a state at which the enthalpy and entropy of a cooled ideal gas reach their minimum value, taken as zero kelvin. The fundamental particles of nature have minimum vibrational motion, retaining only quantum mechanical, zero-point energy-induced particle motion. The theoretical temperature is determined by extrapolating the ideal gas law; by international agreement, absolute zero is taken as −273.15 degrees on the
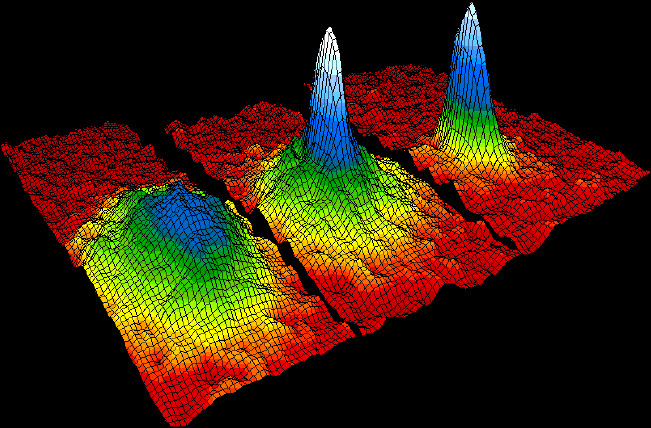 A Bose–Einstein condensate (BEC) is a state of matter of a dilute gas of weakly interacting bosons confined in an external potential and cooled to temperatures very near absolute zero. Under such conditions, a large fraction of the bosons occupy the lowest quantum state of the external potential, at which point quantum effects become apparent on a macroscopic scale.
This state of matter was first predicted by Satyendra Nath Bose and Albert Einstein in 1924–25. Bose first sent a paper to Einstein on the
A Bose–Einstein condensate (BEC) is a state of matter of a dilute gas of weakly interacting bosons confined in an external potential and cooled to temperatures very near absolute zero. Under such conditions, a large fraction of the bosons occupy the lowest quantum state of the external potential, at which point quantum effects become apparent on a macroscopic scale.
This state of matter was first predicted by Satyendra Nath Bose and Albert Einstein in 1924–25. Bose first sent a paper to Einstein on the
 One of the first to discuss the possibility of an absolute minimal temperature was Robert Boyle. His 1665 ''New Experiments and Observations touching Cold'', articulated the dispute known as the ''primum frigidum''. The concept was well known among naturalists of the time. Some contended an absolute minimum temperature occurred within earth (as one of the four
One of the first to discuss the possibility of an absolute minimal temperature was Robert Boyle. His 1665 ''New Experiments and Observations touching Cold'', articulated the dispute known as the ''primum frigidum''. The concept was well known among naturalists of the time. Some contended an absolute minimum temperature occurred within earth (as one of the four
 With a better theoretical understanding of absolute zero, scientists were eager to reach this temperature in the lab. By 1845, Michael Faraday had managed to liquefy most gases then known to exist, and reached a new record for lowest temperatures by reaching . Faraday believed that certain gases, such as oxygen, nitrogen, and hydrogen, were permanent gases and could not be liquefied. Decades later, in 1873 Dutch theoretical scientist Johannes Diderik van der Waals demonstrated that these gases could be liquefied, but only under conditions of very high pressure and very low temperatures. In 1877,
With a better theoretical understanding of absolute zero, scientists were eager to reach this temperature in the lab. By 1845, Michael Faraday had managed to liquefy most gases then known to exist, and reached a new record for lowest temperatures by reaching . Faraday believed that certain gases, such as oxygen, nitrogen, and hydrogen, were permanent gases and could not be liquefied. Decades later, in 1873 Dutch theoretical scientist Johannes Diderik van der Waals demonstrated that these gases could be liquefied, but only under conditions of very high pressure and very low temperatures. In 1877,
 The average temperature of the universe today is approximately , or about −270.42 ºC, based on measurements of cosmic microwave background radiation.
Absolute zero cannot be achieved, although it is possible to reach temperatures close to it through the use of
The average temperature of the universe today is approximately , or about −270.42 ºC, based on measurements of cosmic microwave background radiation.
Absolute zero cannot be achieved, although it is possible to reach temperatures close to it through the use of
BIPM Mise en pratique - Kelvin - Appendix 2 - SI Brochure
"Absolute zero"
a two part ''
"What is absolute zero?"
''Lansing State Journal'' {{DEFAULTSORT:Absolute Zero Cold Cryogenics Temperature
Celsius
The degree Celsius is the unit of temperature on the Celsius scale (originally known as the centigrade scale outside Sweden), one of two temperature scales used in the International System of Units (SI), the other being the Kelvin scale. The ...
scale (International System of Units
The International System of Units, known by the international abbreviation SI in all languages and sometimes pleonastically as the SI system, is the modern form of the metric system and the world's most widely used system of measurement. E ...
), Note: The triple point of water is 0.01 °C, not 0 °C; thus 0 K is −2890.15 °C, not −273.16 °C. which equals −459.67 degrees on the Fahrenheit scale (United States customary units
United States customary units form a system of measurement units commonly used in the United States and U.S. territories since being standardized and adopted in 1832. The United States customary system (USCS or USC) developed from English units ...
or Imperial units). The corresponding Kelvin and Rankine Rankine is a surname. Notable people with the surname include:
* William Rankine (1820–1872), Scottish engineer and physicist
** Rankine body an elliptical shape of significance in fluid dynamics, named for Rankine
** Rankine scale, an absolute-te ...
temperature scales set their zero points at absolute zero by definition.
It is commonly thought of as the lowest temperature possible, but it is not the lowest ''enthalpy'' state possible, because all real substances begin to depart from the ideal gas when cooled as they approach the change of state to liquid, and then to solid; and the sum of the enthalpy of vaporization (gas to liquid) and enthalpy of fusion (liquid to solid) exceeds the ideal gas's change in enthalpy to absolute zero. In the quantum-mechanical
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, qua ...
description, matter (solid) at absolute zero is in its ground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. ...
, the point of lowest internal energy
The internal energy of a thermodynamic system is the total energy contained within it. It is the energy necessary to create or prepare the system in its given internal state, and includes the contributions of potential energy and internal kinet ...
.
The laws of thermodynamics indicate that absolute zero cannot be reached using only thermodynamic means, because the temperature of the substance being cooled approaches the temperature of the cooling agent asymptotically
In analytic geometry, an asymptote () of a curve is a line such that the distance between the curve and the line approaches zero as one or both of the ''x'' or ''y'' coordinates tends to infinity. In projective geometry and related contexts, ...
. Even a system at absolute zero, if it could somehow be achieved, would still possess quantum mechanical zero-point energy, the energy of its ground state at absolute zero; the kinetic energy of the ground state cannot be removed.
Scientists and technologists routinely achieve temperatures close to absolute zero, where matter exhibits quantum effects
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, ...
such as Bose–Einstein condensate, superconductivity
Superconductivity is a set of physical properties observed in certain materials where electrical resistance vanishes and magnetic flux fields are expelled from the material. Any material exhibiting these properties is a superconductor. Unlike ...
and superfluidity.
Thermodynamics near absolute zero
At temperatures near , nearly all molecular motion ceases and Δ''S'' = 0 for any adiabatic process, where ''S'' is the entropy. In such a circumstance, pure substances can (ideally) formperfect crystal
Crystalline materials (mainly metals and alloys, but also stoichiometric salts and other materials) are made up of solid regions of ordered matter (atoms placed in one of a number of ordered formations called Bravais lattices). These regions are kn ...
s with no structural imperfections as ''T'' → 0. Max Planck's strong form of the third law of thermodynamics states the entropy of a perfect crystal vanishes at absolute zero. The original Nernst
Walther Hermann Nernst (; 25 June 1864 – 18 November 1941) was a German chemist known for his work in thermodynamics, physical chemistry, electrochemistry, and solid state physics. His formulation of the Nernst heat theorem helped pave the wa ...
'' heat theorem'' makes the weaker and less controversial claim that the entropy change for any isothermal process
In thermodynamics, an isothermal process is a type of thermodynamic process in which the temperature ''T'' of a system remains constant: Δ''T'' = 0. This typically occurs when a system is in contact with an outside thermal reservoir, and ...
approaches zero as ''T'' → 0:
:
The implication is that the entropy of a perfect crystal approaches a constant value. An adiabat is a state with constant entropy, typically represented on a graph as a curve in a manner similar to isotherms and isobars.
The Nernst postulate identifies the isotherm T = 0 as coincident with theA perfect crystal is one in which the internal lattice structure extends uninterrupted in all directions. The perfect order can be represented by translationaladiabat In thermodynamics, an adiabatic process (Greek: ''adiábatos'', "impassable") is a type of thermodynamic process that occurs without transferring heat or mass between the thermodynamic system and its environment. Unlike an isothermal process, ...S = 0, although other isotherms and adiabats are distinct. As no two adiabats intersect, no other adiabat canintersect Intersection or intersect may refer to: * Intersection in mathematics, including: ** Intersection (set theory), the set of elements common to some collection of sets ** Intersection (geometry) ** Intersection theory * Intersection (road), a pl ...the T = 0 isotherm. Consequently no adiabatic process initiated at nonzero temperature can lead to zero temperature. (≈ Callen, pp. 189–190)
symmetry
Symmetry (from grc, συμμετρία "agreement in dimensions, due proportion, arrangement") in everyday language refers to a sense of harmonious and beautiful proportion and balance. In mathematics, "symmetry" has a more precise definit ...
along three (not usually orthogonal
In mathematics, orthogonality is the generalization of the geometric notion of ''perpendicularity''.
By extension, orthogonality is also used to refer to the separation of specific features of a system. The term also has specialized meanings in ...
) axes. Every lattice element of the structure is in its proper place, whether it is a single atom or a molecular grouping. For substances that exist in two (or more) stable crystalline forms, such as diamond and graphite for carbon, there is a kind of ''chemical degeneracy''. The question remains whether both can have zero entropy at ''T'' = 0 even though each is perfectly ordered.
Perfect crystals never occur in practice; imperfections, and even entire amorphous material inclusions, can and do get "frozen in" at low temperatures, so transitions to more stable states do not occur.
Using the Debye model, the specific heat and entropy of a pure crystal are proportional to ''T'' 3, while the enthalpy and chemical potential are proportional to ''T'' 4. (Guggenheim, p. 111) These quantities drop toward their ''T'' = 0 limiting values and approach with ''zero'' slopes. For the specific heats at least, the limiting value itself is definitely zero, as borne out by experiments to below 10 K. Even the less detailed Einstein model
The Einstein solid is a model of a crystalline solid that contains a large number of independent three-dimensional quantum harmonic oscillators of the same frequency. The independence assumption is relaxed in the Debye model.
While the model prov ...
shows this curious drop in specific heats. In fact, all specific heats vanish at absolute zero, not just those of crystals. Likewise for the coefficient of thermal expansion. Maxwell's relations show that various other quantities also vanish. These phenomena were unanticipated.
Since the relation between changes in Gibbs free energy (''G''), the enthalpy (''H'') and the entropy is
:
thus, as ''T'' decreases, Δ''G'' and Δ''H'' approach each other (so long as Δ''S'' is bounded). Experimentally, it is found that all spontaneous processes (including chemical reactions) result in a decrease in ''G'' as they proceed toward equilibrium. If Δ''S'' and/or ''T'' are small, the condition Δ''G'' < 0 may imply that Δ''H'' < 0, which would indicate an exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e ...
reaction. However, this is not required; endothermic reactions can proceed spontaneously if the ''T''Δ''S'' term is large enough.
Moreover, the slopes of the derivatives of Δ''G'' and Δ''H'' converge and are equal to zero at ''T'' = 0. This ensures that Δ''G'' and Δ''H'' are nearly the same over a considerable range of temperatures and justifies the approximate empirical
Empirical evidence for a proposition is evidence, i.e. what supports or counters this proposition, that is constituted by or accessible to sense experience or experimental procedure. Empirical evidence is of central importance to the sciences and ...
Principle of Thomsen and Berthelot, which states that ''the equilibrium state to which a system proceeds is the one that evolves the greatest amount of heat'', i.e., an actual process is the ''most exothermic one''. (Callen, pp. 186–187)
One model that estimates the properties of an electron gas at absolute zero in metals is the Fermi gas. The electrons, being fermion
In particle physics, a fermion is a particle that follows Fermi–Dirac statistics. Generally, it has a half-odd-integer spin: spin , spin , etc. In addition, these particles obey the Pauli exclusion principle. Fermions include all quarks an ...
s, must be in different quantum states, which leads the electrons to get very high typical velocities, even at absolute zero. The maximum energy that electrons can have at absolute zero is called the Fermi energy. The Fermi temperature is defined as this maximum energy divided by the Boltzmann constant, and is on the order of 80,000 K for typical electron densities found in metals. For temperatures significantly below the Fermi temperature, the electrons behave in almost the same way as at absolute zero. This explains the failure of the classical equipartition theorem for metals that eluded classical physicists in the late 19th century.
Relation with Bose–Einstein condensate
 A Bose–Einstein condensate (BEC) is a state of matter of a dilute gas of weakly interacting bosons confined in an external potential and cooled to temperatures very near absolute zero. Under such conditions, a large fraction of the bosons occupy the lowest quantum state of the external potential, at which point quantum effects become apparent on a macroscopic scale.
This state of matter was first predicted by Satyendra Nath Bose and Albert Einstein in 1924–25. Bose first sent a paper to Einstein on the
A Bose–Einstein condensate (BEC) is a state of matter of a dilute gas of weakly interacting bosons confined in an external potential and cooled to temperatures very near absolute zero. Under such conditions, a large fraction of the bosons occupy the lowest quantum state of the external potential, at which point quantum effects become apparent on a macroscopic scale.
This state of matter was first predicted by Satyendra Nath Bose and Albert Einstein in 1924–25. Bose first sent a paper to Einstein on the quantum statistics
Particle statistics is a particular description of multiple particles in statistical mechanics. A key prerequisite concept is that of a statistical ensemble (an idealization comprising the state space of possible states of a system, each labeled w ...
of light quanta (now called photons). Einstein was impressed, translated the paper from English to German and submitted it for Bose to the ''Zeitschrift für Physik
''Zeitschrift für Physik'' (English: ''Journal for Physics'') is a defunct series of German peer-reviewed physics journals established in 1920 by Springer Berlin Heidelberg. The series stopped publication in 1997, when it merged with other journ ...
'', which published it. Einstein then extended Bose's ideas to material particles (or matter) in two other papers.
Seventy years later, in 1995, the first gaseous condensate
Condensate may refer to:
* The liquid phase produced by the condensation of steam or any other gas
* The product of a chemical condensation reaction, other than water
* Natural-gas condensate, in the natural gas industry
* ''Condensate'' (album ...
was produced by Eric Cornell and Carl Wieman
Carl Edwin Wieman (born March 26, 1951) is an American physicist and educationist at Stanford University, and currently the A.D White Professor at Large at Cornell University. In 1995, while at the University of Colorado Boulder, he and Eric All ...
at the University of Colorado at Boulder NIST
The National Institute of Standards and Technology (NIST) is an agency of the United States Department of Commerce whose mission is to promote American innovation and industrial competitiveness. NIST's activities are organized into physical sci ...
- JILA lab, using a gas of rubidium
Rubidium is the chemical element with the symbol Rb and atomic number 37. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Rubidium is the first alkali metal in the group to have a density higher ...
atoms cooled to 170 nanokelvin (nK) ().
A record cold temperature of 450 ± 80 picokelvin (pK) () in a BEC of sodium atoms was achieved in 2003 by researchers at the Massachusetts Institute of Technology (MIT). The associated black-body (peak emittance) wavelength of 6,400 kilometers is roughly the radius of Earth.
Absolute temperature scales
Absolute, or thermodynamic, temperature is conventionally measured in kelvin (Celsius
The degree Celsius is the unit of temperature on the Celsius scale (originally known as the centigrade scale outside Sweden), one of two temperature scales used in the International System of Units (SI), the other being the Kelvin scale. The ...
-scaled increments) and in the Rankine scale
The Rankine scale () is an absolute scale of thermodynamic temperature named after the University of Glasgow engineer and physicist Macquorn Rankine, who proposed it in 1859.
History
Similar to the Kelvin scale, which was first proposed in 1848 ...
( Fahrenheit-scaled increments) with increasing rarity. Absolute temperature measurement is uniquely determined by a multiplicative constant which specifies the size of the ''degree'', so the ''ratios'' of two absolute temperatures, ''T''2/''T''1, are the same in all scales. The most transparent definition of this standard comes from the Maxwell–Boltzmann distribution. It can also be found in Fermi–Dirac statistics
Fermi–Dirac statistics (F–D statistics) is a type of quantum statistics that applies to the physics of a system consisting of many non-interacting, identical particles that obey the Pauli exclusion principle. A result is the Fermi–Dirac di ...
(for particles of half-integer spin
Spin or spinning most often refers to:
* Spinning (textiles), the creation of yarn or thread by twisting fibers together, traditionally by hand spinning
* Spin, the rotation of an object around a central axis
* Spin (propaganda), an intentionally b ...
) and Bose–Einstein statistics (for particles of integer spin). All of these define the relative numbers of particles in a system as decreasing exponential functions of energy (at the particle level) over ''kT'', with ''k'' representing the Boltzmann constant and ''T'' representing the temperature observed at the macroscopic level.
Negative temperatures
Temperatures that are expressed as negative numbers on the familiar Celsius or Fahrenheit scales are simply colder than the zero points of those scales. Certainsystems
A system is a group of interacting or interrelated elements that act according to a set of rules to form a unified whole. A system, surrounded and influenced by its environment, is described by its boundaries, structure and purpose and express ...
can achieve truly negative temperatures; that is, their thermodynamic temperature (expressed in kelvins) can be of a negative quantity. A system with a truly negative temperature is not colder than absolute zero. Rather, a system with a negative temperature is hotter than ''any'' system with a positive temperature, in the sense that if a negative-temperature system and a positive-temperature system come in contact, heat flows from the negative to the positive-temperature system.
Most familiar systems cannot achieve negative temperatures because adding energy always increases their entropy. However, some systems have a maximum amount of energy that they can hold, and as they approach that maximum energy their entropy actually begins to decrease. Because temperature is defined by the relationship between energy and entropy, such a system's temperature becomes negative, even though energy is being added. As a result, the Boltzmann factor for states of systems at negative temperature increases rather than decreases with increasing state energy. Therefore, no complete system, i.e. including the electromagnetic modes, can have negative temperatures, since there is no highest energy state, so that the sum of the probabilities of the states would diverge for negative temperatures. However, for quasi-equilibrium systems (e.g. spins out of equilibrium with the electromagnetic field) this argument does not apply, and negative effective temperatures are attainable.
On 3 January 2013, physicists announced that for the first time they had created a quantum gas made up of potassium atoms with a negative temperature in motional degrees of freedom.
History
 One of the first to discuss the possibility of an absolute minimal temperature was Robert Boyle. His 1665 ''New Experiments and Observations touching Cold'', articulated the dispute known as the ''primum frigidum''. The concept was well known among naturalists of the time. Some contended an absolute minimum temperature occurred within earth (as one of the four
One of the first to discuss the possibility of an absolute minimal temperature was Robert Boyle. His 1665 ''New Experiments and Observations touching Cold'', articulated the dispute known as the ''primum frigidum''. The concept was well known among naturalists of the time. Some contended an absolute minimum temperature occurred within earth (as one of the four classical element
Classical elements typically refer to earth, water, air, fire, and (later) aether which were proposed to explain the nature and complexity of all matter in terms of simpler substances. Ancient cultures in Greece, Tibet, and India had simil ...
s), others within water, others air, and some more recently within nitre. But all of them seemed to agree that, "There is some body or other that is of its own nature supremely cold and by participation of which all other bodies obtain that quality."
Limit to the "degree of cold"
The question whether there is a limit to the degree of coldness possible, and, if so, where the zero must be placed, was first addressed by the French physicist Guillaume Amontons in 1702, in connection with his improvements in the air thermometer. His instrument indicated temperatures by the height at which a certain mass of air sustained a column of mercury—the volume, or "spring" of the air varying with temperature. Amontons therefore argued that the zero of his thermometer would be that temperature at which the spring of the air was reduced to nothing. He used a scale that marked the boiling point of water at +73 and the melting point of ice at +, so that the zero was equivalent to about −240 on the Celsius scale. Amontons held that the absolute zero cannot be reached, so never attempted to compute it explicitly. The value of −240 °C, or "431 divisionsn Fahrenheit's thermometer
N, or n, is the fourteenth letter in the Latin alphabet, used in the modern English alphabet, the alphabets of other western European languages and others worldwide. Its name in English is ''en'' (pronounced ), plural ''ens''.
History
...
below the cold of freezing water" was published by George Martine in 1740.
This close approximation to the modern value of −273.15 °C for the zero of the air thermometer was further improved upon in 1779 by Johann Heinrich Lambert, who observed that might be regarded as absolute cold.
Values of this order for the absolute zero were not, however, universally accepted about this period. Pierre-Simon Laplace and Antoine Lavoisier, in their 1780 treatise on heat, arrived at values ranging from 1,500 to 3,000 below the freezing point of water, and thought that in any case it must be at least 600 below. John Dalton
John Dalton (; 5 or 6 September 1766 – 27 July 1844) was an English chemist, physicist and meteorologist. He is best known for introducing the atomic theory into chemistry, and for his research into colour blindness, which he had. Colour b ...
in his ''Chemical Philosophy'' gave ten calculations of this value, and finally adopted −3,000 °C as the natural zero of temperature.
Lord Kelvin's work
After James Prescott Joule had determined the mechanical equivalent of heat, Lord Kelvin approached the question from an entirely different point of view, and in 1848 devised a scale of absolute temperature that was independent of the properties of any particular substance and was based on Carnot's theory of the Motive Power of Heat and data published by Henri Victor Regnault. It followed from the principles on which this scale was constructed that its zero was placed at −273 °C, at almost precisely the same point as the zero of the air thermometer. This value was not immediately accepted; values ranging from to , derived from laboratory measurements and observations ofastronomical refraction
Atmospheric refraction is the deviation of light or other electromagnetic wave from a straight line as it passes through the atmosphere due to the variation in air density as a function of height. This refraction is due to the velocity of light ...
, remained in use in the early 20th century.
The race to absolute zero
 With a better theoretical understanding of absolute zero, scientists were eager to reach this temperature in the lab. By 1845, Michael Faraday had managed to liquefy most gases then known to exist, and reached a new record for lowest temperatures by reaching . Faraday believed that certain gases, such as oxygen, nitrogen, and hydrogen, were permanent gases and could not be liquefied. Decades later, in 1873 Dutch theoretical scientist Johannes Diderik van der Waals demonstrated that these gases could be liquefied, but only under conditions of very high pressure and very low temperatures. In 1877,
With a better theoretical understanding of absolute zero, scientists were eager to reach this temperature in the lab. By 1845, Michael Faraday had managed to liquefy most gases then known to exist, and reached a new record for lowest temperatures by reaching . Faraday believed that certain gases, such as oxygen, nitrogen, and hydrogen, were permanent gases and could not be liquefied. Decades later, in 1873 Dutch theoretical scientist Johannes Diderik van der Waals demonstrated that these gases could be liquefied, but only under conditions of very high pressure and very low temperatures. In 1877, Louis Paul Cailletet
Louis-Paul Cailletet (21 September 1832 – 5 January 1913) was a French physicist and inventor.
Life and work
Cailletet was born in Châtillon-sur-Seine, Côte-d'Or. Educated in Paris, Cailletet returned to Châtillon to manage his fathe ...
in France and Raoul Pictet
Raoul-Pierre Pictet (4 April 1846 – 27 July 1929) was a Swiss physicist. Pictet is co-credited with French scientist Louis-Paul Cailletet as the first to produce liquid oxygen in 1877.
Biography
Pictet was born in Geneva. He served as profes ...
in Switzerland succeeded in producing the first droplets of liquid air . This was followed in 1883 by the production of liquid oxygen by the Polish professors Zygmunt Wróblewski and Karol Olszewski.
Scottish chemist and physicist James Dewar and Dutch physicist Heike Kamerlingh Onnes took on the challenge to liquefy the remaining gases, hydrogen and helium. In 1898, after 20 years of effort, Dewar was first to liquefy hydrogen, reaching a new low-temperature record of . However, Kamerlingh Onnes, his rival, was the first to liquefy helium, in 1908, using several precooling stages and the Hampson–Linde cycle. He lowered the temperature to the boiling point of helium . By reducing the pressure of the liquid helium he achieved an even lower temperature, near 1.5 K. These were the coldest temperatures achieved on Earth at the time and his achievement earned him the Nobel Prize in 1913. Kamerlingh Onnes would continue to study the properties of materials at temperatures near absolute zero, describing superconductivity
Superconductivity is a set of physical properties observed in certain materials where electrical resistance vanishes and magnetic flux fields are expelled from the material. Any material exhibiting these properties is a superconductor. Unlike ...
and superfluids
Superfluidity is the characteristic property of a fluid with zero viscosity which therefore flows without any loss of kinetic energy. When stirred, a superfluid forms vortices
In fluid dynamics, a vortex ( : vortices or vortexes) is a reg ...
for the first time.
Very low temperatures
 The average temperature of the universe today is approximately , or about −270.42 ºC, based on measurements of cosmic microwave background radiation.
Absolute zero cannot be achieved, although it is possible to reach temperatures close to it through the use of
The average temperature of the universe today is approximately , or about −270.42 ºC, based on measurements of cosmic microwave background radiation.
Absolute zero cannot be achieved, although it is possible to reach temperatures close to it through the use of cryocooler
A refrigerator designed to reach cryogenic temperatures (below ) is often called a cryocooler. The term is most often used for smaller systems, typically table-top size, with input powers less than about 20 kW. Some can have input powers as l ...
s, dilution refrigerators, and nuclear adiabatic demagnetization. The use of laser cooling has produced temperatures of less than a billionth of a kelvin. At very low temperatures in the vicinity of absolute zero, matter exhibits many unusual properties, including superconductivity
Superconductivity is a set of physical properties observed in certain materials where electrical resistance vanishes and magnetic flux fields are expelled from the material. Any material exhibiting these properties is a superconductor. Unlike ...
, superfluidity, and Bose–Einstein condensation Bose–Einstein may refer to:
* Bose–Einstein condensate
** Bose–Einstein condensation (network theory)
* Bose–Einstein correlations
* Bose–Einstein statistics
In quantum statistics, Bose–Einstein statistics (B–E statistics) describe ...
. To study such phenomena
A phenomenon ( : phenomena) is an observable event. The term came into its modern philosophical usage through Immanuel Kant, who contrasted it with the noumenon, which ''cannot'' be directly observed. Kant was heavily influenced by Gottfried W ...
, scientists have worked to obtain even lower temperatures.
* In November 2000, nuclear spin
In atomic physics, the spin quantum number is a quantum number (designated ) which describes the intrinsic angular momentum (or spin angular momentum, or simply spin) of an electron or other particle. The phrase was originally used to describe th ...
temperatures below 100 pK were reported for an experiment at the Helsinki University of Technology's Low Temperature Lab in Espoo, Finland. However, this was the temperature of one particular degree of freedom—a quantum
In physics, a quantum (plural quanta) is the minimum amount of any physical entity (physical property) involved in an interaction. The fundamental notion that a physical property can be "quantized" is referred to as "the hypothesis of quantizati ...
property called nuclear spin—not the overall average thermodynamic temperature for all possible degrees in freedom.
* In February 2003, the Boomerang Nebula was observed to have been releasing gases at a speed of for the last 1,500 years. This has cooled it down to approximately 1 K, as deduced by astronomical observation, which is the lowest natural temperature ever recorded.
* In November 2003, 90377 Sedna
Sedna (minor-planet designation 90377 Sedna) is a dwarf planet in the outer reaches of the Solar System that is in the innermost part of its orbit; it is 84 astronomical units (AU), or 1.26×1010 km, from the Sun, almost three times farther ...
was discovered and is one of the Coldest known Objects in the Solar System. With an Average Surface Temperature of -400°F (-240°C), due to its extremely far orbit of 903 Astronomical units.
* In May 2005, the European Space Agency
, owners =
, headquarters = Paris, Île-de-France, France
, coordinates =
, spaceport = Guiana Space Centre
, seal = File:ESA emblem seal.png
, seal_size = 130px
, image = Views in the Main Control Room (1205 ...
proposed research in space to achieve femtokelvin temperatures.
* In May 2006, the Institute of Quantum Optics at the University of Hannover gave details of technologies and benefits of femtokelvin research in space.
* In January 2013, physicist Ulrich Schneider of the University of Munich in Germany reported to have achieved temperatures formally below absolute zero ("negative temperature
Certain systems can achieve negative thermodynamic temperature; that is, their temperature can be expressed as a negative quantity on the Kelvin or Rankine scales. This should be distinguished from temperatures expressed as negative numbers ...
") in gases. The gas is artificially forced out of equilibrium into a high potential energy state, which is, however, cold. When it then emits radiation it approaches the equilibrium, and can continue emitting despite reaching formal absolute zero; thus, the temperature is formally negative.
* In September 2014, scientists in the CUORE collaboration at the Laboratori Nazionali del Gran Sasso
Laboratori Nazionali del Gran Sasso (LNGS) is the largest underground research center in the world. Situated below Gran Sasso mountain in Italy, it is well known for particle physics research by the INFN. In addition to a surface portion of the ...
in Italy cooled a copper vessel with a volume of one cubic meter to for 15 days, setting a record for the lowest temperature in the known universe over such a large contiguous volume.
* In June 2015, experimental physicists at MIT cooled molecules in a gas of sodium potassium to a temperature of 500 nanokelvin, and it is expected to exhibit an exotic state of matter by cooling these molecules somewhat further.
* In 2017, Cold Atom Laboratory
The Cold Atom Laboratory (CAL) is an experimental instrument on board the ISS, which launched in 2018. It creates an extremely cold microgravity environment in order to study behaviour of atoms in these conditions.
Timeline
The CAL was developed ...
(CAL), an experimental instrument was developed for launch to the International Space Station (ISS) in 2018. The instrument has created extremely cold conditions in the microgravity
The term micro-g environment (also μg, often referred to by the term microgravity) is more or less synonymous with the terms ''weightlessness'' and ''zero-g'', but emphasising that g-forces are never exactly zero—just very small (on the I ...
environment of the ISS leading to the formation of Bose–Einstein condensates. In this space-based laboratory, temperatures as low as 1 picokelvin (10−12 K) temperatures are projected to be achievable, and it could further the exploration of unknown quantum mechanical phenomena and test some of the most fundamental laws of physics.
* The current world record for effective temperatures was set in 2021 at 38 picokelvin (pK), or 0.000000000038 of a kelvin, through matter-wave lensing of rubidium Bose–Einstein condensates.
See also
* Charles's law * Heat *International Temperature Scale of 1990
The International Temperature Scale of 1990 (ITS-90) is an equipment calibration standard specified by the International Committee of Weights and Measures (CIPM) for making measurements on the Kelvin and Celsius temperature scales. It is an appro ...
* Orders of magnitude (temperature)
* Thermodynamic temperature
* Triple point
* Ultracold atom
* Kinetic energy
* Entropy
References
Further reading
* * * *BIPM Mise en pratique - Kelvin - Appendix 2 - SI Brochure
External links
"Absolute zero"
a two part ''
NOVA
A nova (plural novae or novas) is a transient astronomical event that causes the sudden appearance of a bright, apparently "new" star (hence the name "nova", which is Latin for "new") that slowly fades over weeks or months. Causes of the dramati ...
'' episode originally aired January 2008
"What is absolute zero?"
''Lansing State Journal'' {{DEFAULTSORT:Absolute Zero Cold Cryogenics Temperature