|
Hampson–Linde Cycle
The Hampson–Linde cycle is a process for the liquefaction of gases, especially for air separation. William Hampson and Carl von Linde independently filed for patents of the cycle in 1895: Hampson on 23 May 1895 and Linde on 5 June 1895. The Hampson–Linde cycle introduced regenerative cooling, a positive-feedback cooling system. The heat exchanger arrangement permits an absolute temperature difference (e.g. J–T cooling for air) to go beyond a single stage of cooling and can reach the low temperatures required to liquefy "fixed" gases. The Hampson–Linde cycle differs from the Siemens cycle only in the expansion step. Whereas the Siemens cycle has the gas do external work to reduce its temperature, the Hampson–Linde cycle relies solely on the Joule–Thomson effect In thermodynamics, the Joule–Thomson effect (also known as the Joule–Kelvin effect or Kelvin–Joule effect) describes the temperature change of a ''real'' gas or liquid (as differentiated from an idea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linde Cycle 1895 -1903 Patent Cryo
Linde may refer to: Places *Lindes and Ramsberg Mountain District, a former district in Sweden, see Lindesberg Municipality *Lipka, Złotów County, a village in Poland, called Linde before World War II Rivers *Linde (Tollense), a river of Mecklenburg-Vorpommern, Germany *Linde (Lena), a river in Sakha Republic, Russia Other uses *Linde (surname) *Linde plc, an international industrial gases company *Linde Hydraulics, a manufacturer of heavy duty drive systems *Mercedes-Benz Championship (European Tour), formerly the Linde German Masters, a professional golf tournament played in Germany People * Fedor Linde, a russian revolutionary See also *Linde–Buzo–Gray algorithm, an algorithm in vector quantization to derive a good codebook *Lind (other) *Linden (other) * Lindner *Lindemann (Lindeman Lindeman is a German, Dutch, Norwegian and Swedish surname. Geographical distribution As of 2014, 58.8% of all known bearers of the surname ''Lindeman'' were residen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
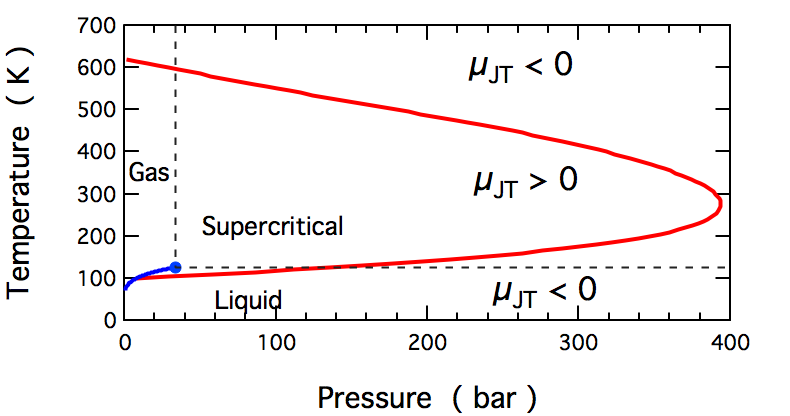
Joule–Thomson Effect
In thermodynamics, the Joule–Thomson effect (also known as the Joule–Kelvin effect or Kelvin–Joule effect) describes the temperature change of a ''real'' gas or liquid (as differentiated from an ideal gas) when it is forced through a valve or porous plug while keeping it insulated so that no heat is exchanged with the environment. This procedure is called a ''throttling process'' or ''Joule–Thomson process''. At room temperature, all gases except hydrogen, helium, and neon cool upon expansion by the Joule–Thomson process when being throttled through an orifice; these three gases experience the same effect but only at lower temperatures. Most liquids such as hydraulic oils will be warmed by the Joule–Thomson throttling process. The gas-cooling throttling process is commonly exploited in refrigeration processes such as liquefiers in air separation industrial process. In hydraulics, the warming effect from Joule–Thomson throttling can be used to find internal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Industrial Gases
Industrial gases are the gaseous materials that are manufactured for use in industry. The principal gases provided are nitrogen, oxygen, carbon dioxide, argon, hydrogen, helium and acetylene, although many other gases and mixtures are also available in gas cylinders. The industry producing these gases is also known as industrial gas, which is seen as also encompassing the supply of equipment and technology to produce and use the gases. Their production is a part of the wider chemical Industry (where industrial gases are often seen as "specialty chemicals"). Industrial gases are used in a wide range of industries, which include oil and gas, petrochemicals, chemicals, power, mining, steelmaking, metals, environmental protection, medicine, pharmaceuticals, biotechnology, food, water, fertilizers, nuclear power, electronics and aerospace. Industrial gas is sold to other industrial enterprises; typically comprising large orders to corporate industrial clients, covering a size range fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cryogenics
In physics, cryogenics is the production and behaviour of materials at very low temperatures. The 13th IIR International Congress of Refrigeration (held in Washington DC in 1971) endorsed a universal definition of “cryogenics” and “cryogenic” by accepting a threshold of 120 K (or –153 °C) to distinguish these terms from the conventional refrigeration. This is a logical dividing line, since the normal boiling points of the so-called permanent gases (such as helium, hydrogen, neon, nitrogen, oxygen, and normal air) lie below 120K while the Freon refrigerants, hydrocarbons, and other common refrigerants have boiling points above 120K. The U.S. National Institute of Standards and Technology considers the field of cryogenics as that involving temperatures below -153 Celsius (120K; -243.4 Fahrenheit) Discovery of superconducting materials with critical temperatures significantly above the boiling point of nitrogen has provided new interest in reliable, low cost method ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamic Cycles
A thermodynamic cycle consists of a linked sequence of thermodynamic processes that involve transfer of heat and work into and out of the system, while varying pressure, temperature, and other state variables within the system, and that eventually returns the system to its initial state. In the process of passing through a cycle, the working fluid (system) may convert heat from a warm source into useful work, and dispose of the remaining heat to a cold sink, thereby acting as a heat engine. Conversely, the cycle may be reversed and use work to move heat from a cold source and transfer it to a warm sink thereby acting as a heat pump. If at every point in the cycle the system is in thermodynamic equilibrium, the cycle is reversible. Whether carried out reversible or irreversibly, the net entropy change of the system is zero, as entropy is a state function. During a closed cycle, the system returns to its original thermodynamic state of temperature and pressure. Process quantities ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Countercurrent Exchange
Countercurrent exchange is a mechanism occurring in nature and mimicked in industry and engineering, in which there is a crossover of some property, usually heat or some chemical, between two flowing bodies flowing in opposite directions to each other. The flowing bodies can be liquids, gases, or even solid powders, or any combination of those. For example, in a distillation column, the vapors bubble up through the downward flowing liquid while exchanging both heat and mass. The maximum amount of heat or mass transfer that can be obtained is higher with countercurrent than co-current (parallel) exchange because countercurrent maintains a slowly declining difference or gradient (usually temperature or concentration difference). In cocurrent exchange the initial gradient is higher but falls off quickly, leading to wasted potential. For example, in the adjacent diagram, the fluid being heated (exiting top) has a higher exiting temperature than the cooled fluid (exiting bottom) that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Work (physics)
In physics, work is the energy transferred to or from an object via the application of force along a displacement. In its simplest form, for a constant force aligned with the direction of motion, the work equals the product of the force strength and the distance traveled. A force is said to do ''positive work'' if when applied it has a component in the direction of the displacement of the point of application. A force does ''negative work'' if it has a component opposite to the direction of the displacement at the point of application of the force. For example, when a ball is held above the ground and then dropped, the work done by the gravitational force on the ball as it falls is positive, and is equal to the weight of the ball (a force) multiplied by the distance to the ground (a displacement). If the ball is thrown upwards, the work done by its weight is negative, and is equal to the weight multiplied by the displacement in the upwards direction. When the force is consta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquefaction Of Gases
Liquefaction of gases is physical conversion of a gas into a liquid state ( condensation). The liquefaction of gases is a complicated process that uses various compressions and expansions to achieve high pressures and very low temperatures, using, for example, turboexpanders. Uses Liquefaction processes are used for scientific, industrial and commercial purposes. Many gases can be put into a liquid state at normal atmospheric pressure by simple cooling; a few, such as carbon dioxide, require pressurization as well. Liquefaction is used for analyzing the fundamental properties of gas molecules (intermolecular forces), or for the storage of gases, for example: LPG, and in refrigeration and air conditioning. There the gas is liquefied in the '' condenser'', where the heat of vaporization is released, and evaporated in the ''evaporator,'' where the heat of vaporization is absorbed. Ammonia was the first such refrigerant, and is still in widespread use in industrial refrigeration, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Siemens Cycle
The Siemens cycle is a technique used to cool or liquefy gases. A gas is compressed, leading to an increase in its temperature due to the directly proportional relationship between temperature and pressure (as stated by Gay-Lussac's law). The compressed gas is then cooled by a heat exchanger and decompressed, resulting in a (possibly condensed) gas that is colder than the original at the same pressure. Carl Wilhelm Siemens patented the Siemens cycle in 1857. In the Siemens cycle the gas is: :1. Heated – by compressing the gas – adding external energy into the gas, to give it what is needed for running through the cycle :2. Cooled – by immersing the gas in a cooler environment, losing some of its heat (and energy) :3. Cooled through heat exchanger with returning gas from next (and last stage) :4. Cooled further by expanding the gas and doing work, removing heat (and energy) The gas which is now at its coolest in the current cycle, is recycled and sent back to be – :5. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Exchanger
A heat exchanger is a system used to transfer heat between a source and a working fluid. Heat exchangers are used in both cooling and heating processes. The fluids may be separated by a solid wall to prevent mixing or they may be in direct contact. They are widely used in space heating, refrigeration, air conditioning, power stations, chemical plants, petrochemical plants, petroleum refineries, natural-gas processing, and sewage treatment. The classic example of a heat exchanger is found in an internal combustion engine in which a circulating fluid known as engine coolant flows through radiator coils and air flows past the coils, which cools the coolant and heats the incoming air. Another example is the heat sink, which is a passive heat exchanger that transfers the heat generated by an electronic or a mechanical device to a fluid medium, often air or a liquid coolant. Flow arrangement Image:Heat_exc_1-1.svg, Fig. 1: Shell and tube heat exchanger, single pass (1–1 parallel f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |