 |
Third Law Of Thermodynamics
The third law of thermodynamics states, regarding the properties of closed systems in thermodynamic equilibrium: This constant value cannot depend on any other parameters characterizing the closed system, such as pressure or applied magnetic field. At absolute zero (zero kelvins) the system must be in a state with the minimum possible energy. Entropy is related to the number of accessible microstates, and there is typically one unique state (called the ground state) with minimum energy. In such a case, the entropy at absolute zero will be exactly zero. If the system does not have a well-defined order (if its order is glassy, for example), then there may remain some finite entropy as the system is brought to very low temperatures, either because the system becomes locked into a configuration with non-minimal energy or because the minimum energy state is non-unique. The constant value is called the residual entropy of the system. The entropy is essentially a state-function meaning ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Thermodynamic Equilibrium
Thermodynamic equilibrium is an axiomatic concept of thermodynamics. It is an internal state of a single thermodynamic system, or a relation between several thermodynamic systems connected by more or less permeable or impermeable walls. In thermodynamic equilibrium, there are no net macroscopic flows of matter nor of energy within a system or between systems. In a system that is in its own state of internal thermodynamic equilibrium, no macroscopic change occurs. Systems in mutual thermodynamic equilibrium are simultaneously in mutual thermal, mechanical, chemical, and radiative equilibria. Systems can be in one kind of mutual equilibrium, while not in others. In thermodynamic equilibrium, all kinds of equilibrium hold at once and indefinitely, until disturbed by a thermodynamic operation. In a macroscopic equilibrium, perfectly or almost perfectly balanced microscopic exchanges occur; this is the physical explanation of the notion of macroscopic equilibrium. A thermodyn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Figure Showing Entropy At 0 K
Figure may refer to: General *A shape, drawing, depiction, or geometric configuration * Figure (wood), wood appearance * Figure (music), distinguished from musical motif * Noise figure, in telecommunication * Dance figure, an elementary dance pattern *A person's figure, human physical appearance Arts *Figurine, a miniature statuette representation of a creature *Action figure, a posable jointed solid plastic character figurine *Figure painting, realistic representation, especially of the human form *Figure drawing * Model figure, a scale model of a creature Writing *figure, in writing, a type of floating block (text, table, or graphic separate from the main text) *Figure of speech, also called a rhetorical figure * Christ figure, a type of character * in typesetting, text figures and lining figures Accounting *Figure, a synonym for number *Significant figures in a decimal number Science *Figure of the Earth, the size and shape of the Earth in geodesy Sports *Figure (hors ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Edward A
Edward is an English given name. It is derived from the Anglo-Saxon name ''Ēadweard'', composed of the elements '' ēad'' "wealth, fortune; prosperous" and '' weard'' "guardian, protector”. History The name Edward was very popular in Anglo-Saxon England, but the rule of the Norman and Plantagenet dynasties had effectively ended its use amongst the upper classes. The popularity of the name was revived when Henry III named his firstborn son, the future Edward I, as part of his efforts to promote a cult around Edward the Confessor, for whom Henry had a deep admiration. Variant forms The name has been adopted in the Iberian peninsula since the 15th century, due to Edward, King of Portugal, whose mother was English. The Spanish/Portuguese forms of the name are Eduardo and Duarte. Other variant forms include French Édouard, Italian Edoardo and Odoardo, German, Dutch, Czech and Romanian Eduard and Scandinavian Edvard. Short forms include Ed, Eddy, Eddie, Ted, Teddy and Ned. Peop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
|
Can T=0 Be Reached
Can may refer to: Containers * Aluminum can * Drink can * Oil can * Steel and tin cans * Trash can * Petrol can * Metal can (other) Music * Can (band), West Germany, 1968 ** ''Can'' (album), 1979 * Can (South Korean band) Other * Can (name), Turkish and Circassian given name and surname * Can (verb) * Canning of food * River Can, Essex, UK * Canada Canada is a country in North America. Its ten provinces and three territories extend from the Atlantic Ocean to the Pacific Ocean and northward into the Arctic Ocean, covering over , making it the world's second-largest country by tota ... * Tomato can (sports idiom) See also * CAN (other) * Cann (other) * Cans (other) * Kan (other) {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Quantum Spin Liquid
In condensed matter physics, a quantum spin liquid is a phase of matter that can be formed by interacting quantum spins in certain magnetic materials. Quantum spin liquids (QSL) are generally characterized by their long-range quantum entanglement, fractionalized excitations, and absence of ordinary magnetic order. The quantum spin liquid state was first proposed by physicist Phil Anderson in 1973 as the ground state for a system of spins on a triangular lattice that interact antiferromagnetically with their nearest neighbors, i.e. neighboring spins seek to be aligned in opposite directions. Quantum spin liquids generated further interest when in 1987 Anderson proposed a theory that described high temperature superconductivity in terms of a disordered spin-liquid state. Basic properties The simplest kind of magnetic phase is a paramagnet, where each individual spin behaves independently of the rest, just like atoms in an ideal gas. This highly disordered phase is the gen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Spin Glass
In condensed matter physics, a spin glass is a magnetic state characterized by randomness, besides cooperative behavior in freezing of spins at a temperature called 'freezing temperature' ''Tf''. In ferromagnetic solids, component atoms' magnetic spins all align in the same direction. Spin glass when contrasted with a ferromagnet is defined as " disordered" magnetic state in which spins are aligned randomly or without a regular pattern and the couplings too are random. The term "glass" comes from an analogy between the ''magnetic'' disorder in a spin glass and the ''positional'' disorder of a conventional, chemical glass, e.g., a window glass. In window glass or any amorphous solid the atomic bond structure is highly irregular; in contrast, a crystal has a uniform pattern of atomic bonds. In ferromagnetic solids, magnetic spins all align in the same direction; this is analogous to a crystal's lattice-based structure. The individual atomic bonds in a spin glass are a m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
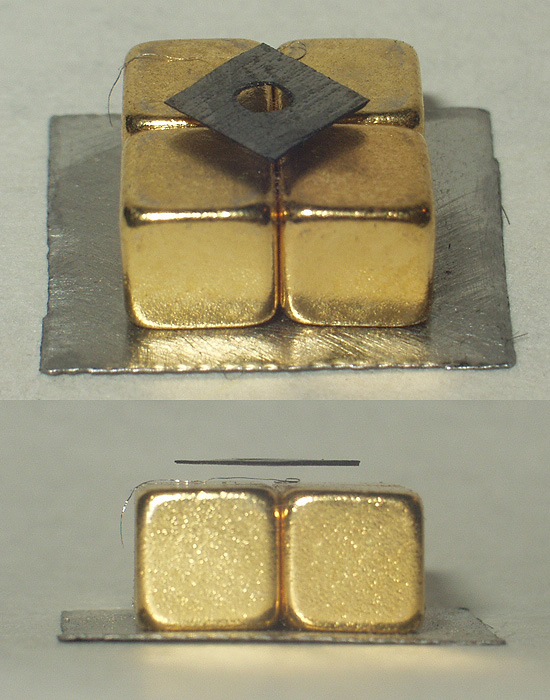 |
Diamagnetic
Diamagnetic materials are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted by a magnetic field. Diamagnetism is a quantum mechanical effect that occurs in all materials; when it is the only contribution to the magnetism, the material is called diamagnetic. In paramagnetic and ferromagnetic substances, the weak diamagnetic force is overcome by the attractive force of magnetic dipoles in the material. The magnetic permeability of diamagnetic materials is less than the permeability of vacuum, ''μ''0. In most materials, diamagnetism is a weak effect which can be detected only by sensitive laboratory instruments, but a superconductor acts as a strong diamagnet because it repels a magnetic field entirely from its interior. Diamagnetism was first discovered when Anton Brugmans observed in 1778 that bismuth was repel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Antiferromagnetic
In materials that exhibit antiferromagnetism, the magnetic moments of atoms or molecules, usually related to the spins of electrons, align in a regular pattern with neighboring spins (on different sublattices) pointing in opposite directions. This is, like ferromagnetism and ferrimagnetism, a manifestation of ordered magnetism. The phenomenon of antiferromagnetism was first introduced by Lev Landau in 1933. Generally, antiferromagnetic order may exist at sufficiently low temperatures, but vanishes at and above the Néel temperature – named after Louis Néel, who had first identified this type of magnetic ordering. Above the Néel temperature, the material is typically paramagnetic. Measurement When no external field is applied, the antiferromagnetic structure corresponds to a vanishing total magnetization. In an external magnetic field, a kind of ferrimagnetic behavior may be displayed in the antiferromagnetic phase, with the absolute value of one of the sublattice magne ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
Ferromagnetic
Ferromagnetism is a property of certain materials (such as iron) which results in a large observed magnetic permeability, and in many cases a large magnetic coercivity allowing the material to form a permanent magnet. Ferromagnetic materials are the familiar metals noticeably attracted to a magnet, a consequence of their large magnetic permeability. Magnetic permeability describes the induced magnetization of a material due to the presence of an ''external'' magnetic field, and it is this temporarily induced magnetization inside a steel plate, for instance, which accounts for its attraction to the permanent magnet. Whether or not that steel plate acquires a permanent magnetization itself, depends not only on the strength of the applied field, but on the so-called coercivity of that material, which varies greatly among ferromagnetic materials. In physics, several different types of material magnetism are distinguished. Ferromagnetism (along with the similar effect ferrimagnet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
Ice Ih
Photograph showing details of an ice cube under magnification. Ice Ih is the form of ice commonly seen on Earth. Phase space of ice Ih with respect to other ice phases. Ice Ih (hexagonal ice crystal) (pronounced: ice one h, also known as ice-phase-one) is the hexagonal crystal form of ordinary ice, or frozen water. Virtually all ice in the biosphere is ice Ih, with the exception only of a small amount of ice Ic that is occasionally present in the upper atmosphere. Ice Ih exhibits many peculiar properties that are relevant to the existence of life and regulation of global climate. For a description of these properties, see ''Ice'', which deals primarily with ice Ih. The crystal structure is characterized by the oxygen atoms forming Hexagonal crystal family, hexagonal symmetry with near tetrahedral bonding angles. Ice Ih is stable down to , as evidenced by x-ray diffraction and extremely high resolution thermal expansion measurements. Ice Ih is also stable under applied pres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
|
 |
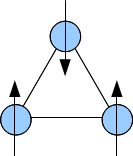
Geometrical Frustration
In condensed matter physics, the term geometrical frustration (or in short: frustration) refers to a phenomenon where atoms tend to stick to non-trivial positions or where, on a regular crystal lattice, conflicting inter-atomic forces (each one favoring rather simple, but different structures) lead to quite complex structures. As a consequence of the frustration in the geometry or in the forces, a plenitude of distinct ground states may result at zero temperature, and usual thermal ordering may be suppressed at higher temperatures. Much studied examples are amorphous materials, glasses, or dilute magnets. The term ''frustration'', in the context of magnetic systems, has been introduced by Gerard Toulouse in 1977. Frustrated magnetic systems had been studied even before. Early work includes a study of the Ising model on a triangular lattice with nearest-neighbor spins coupled antiferromagnetically, by G. H. Wannier, published in 1950. Related features occur in magnets with ''com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |