Wrought Ironwork on:
[Wikipedia]
[Google]
[Amazon]
Wrought iron is an iron alloy with a very low carbon content (less than 0.08%) in contrast to that of cast iron (2.1% to 4%). It is a semi-fused mass of iron with fibrous
 Wrought iron has been used for many centuries, and is the "iron" that is referred to throughout Western history. The other form of iron, cast iron, was in use in China since ancient times but was not introduced into Western Europe until the 15th century; even then, due to its brittleness, it could be used for only a limited number of purposes. Throughout much of the Middle Ages, iron was produced by the direct reduction of ore in manually operated
Wrought iron has been used for many centuries, and is the "iron" that is referred to throughout Western history. The other form of iron, cast iron, was in use in China since ancient times but was not introduced into Western Europe until the 15th century; even then, due to its brittleness, it could be used for only a limited number of purposes. Throughout much of the Middle Ages, iron was produced by the direct reduction of ore in manually operated
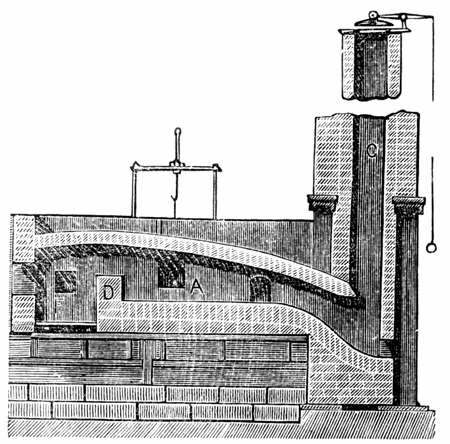 A number of processes for making wrought iron without charcoal were devised as the Industrial Revolution began during the latter half of the 18th century. The most successful of those was puddling, using a puddling furnace (a variety of the reverberatory furnace), which was invented by Henry Cort in 1784. It was later improved by others including Joseph Hall, who was the first to add iron oxide to the charge. In that type of furnace, the metal does not come into contact with the fuel, and so is not contaminated by its impurities . The heat of the combustion products pass over the surface of the puddle and the roof of the furnace reverberates (reflects) the heat onto the metal puddle on the fire bridge of the furnace.
Unless the raw material used is white cast iron, the pig iron or other raw product of the puddling first had to be refined into
A number of processes for making wrought iron without charcoal were devised as the Industrial Revolution began during the latter half of the 18th century. The most successful of those was puddling, using a puddling furnace (a variety of the reverberatory furnace), which was invented by Henry Cort in 1784. It was later improved by others including Joseph Hall, who was the first to add iron oxide to the charge. In that type of furnace, the metal does not come into contact with the fuel, and so is not contaminated by its impurities . The heat of the combustion products pass over the surface of the puddle and the roof of the furnace reverberates (reflects) the heat onto the metal puddle on the fire bridge of the furnace.
Unless the raw material used is white cast iron, the pig iron or other raw product of the puddling first had to be refined into
 The slag inclusions, or stringers, in wrought iron give it properties not found in other forms of ferrous metal. There are approximately 250,000 inclusions per square inch. A fresh fracture shows a clear bluish color with a high silky luster and fibrous appearance.
Wrought iron lacks the carbon content necessary for hardening through heat treatment, but in areas where steel was uncommon or unknown, tools were sometimes cold-worked (hence cold iron) to harden them. An advantage of its low carbon content is its excellent weldability. Furthermore, sheet wrought iron cannot bend as much as steel sheet metal when cold worked. Wrought iron can be melted and cast, however the product is no longer wrought iron, since the slag stringers characteristic of wrought iron disappear on melting, so the product resembles impure, cast, Bessemer steel. There is no engineering advantage as compared to cast iron or steel, both of which are cheaper.
Due to the variations in iron ore origin and iron manufacture, wrought iron can be inferior or superior in corrosion resistance, compared to other iron alloys. There are many mechanisms behind its corrosion resistance. Chilton and Evans found that nickel enrichment bands reduce corrosion. They also found that in puddled, forged, and piled iron, the working-over of the metal spread out copper, nickel, and tin impurities that produce electrochemical conditions that slow down corrosion. The slag inclusions have been shown to disperse corrosion to an even film, enabling the iron to resist pitting. Another study has shown that slag inclusions are pathways to corrosion. Other studies show that sulfur in the wrought iron decreases corrosion resistance, while phosphorus increases corrosion resistance. Chloride ions also decrease wrought iron's corrosion resistance.
Wrought iron may be welded in the same manner as mild steel, but the presence of oxide or inclusions will give defective results.
The material has a rough surface, so it can hold platings and coatings better. For instance, a galvanic zinc finish applied to wrought iron is approximately 25–40% thicker than the same finish on steel. In Table 1, the chemical composition of wrought iron is compared to that of pig iron and
The slag inclusions, or stringers, in wrought iron give it properties not found in other forms of ferrous metal. There are approximately 250,000 inclusions per square inch. A fresh fracture shows a clear bluish color with a high silky luster and fibrous appearance.
Wrought iron lacks the carbon content necessary for hardening through heat treatment, but in areas where steel was uncommon or unknown, tools were sometimes cold-worked (hence cold iron) to harden them. An advantage of its low carbon content is its excellent weldability. Furthermore, sheet wrought iron cannot bend as much as steel sheet metal when cold worked. Wrought iron can be melted and cast, however the product is no longer wrought iron, since the slag stringers characteristic of wrought iron disappear on melting, so the product resembles impure, cast, Bessemer steel. There is no engineering advantage as compared to cast iron or steel, both of which are cheaper.
Due to the variations in iron ore origin and iron manufacture, wrought iron can be inferior or superior in corrosion resistance, compared to other iron alloys. There are many mechanisms behind its corrosion resistance. Chilton and Evans found that nickel enrichment bands reduce corrosion. They also found that in puddled, forged, and piled iron, the working-over of the metal spread out copper, nickel, and tin impurities that produce electrochemical conditions that slow down corrosion. The slag inclusions have been shown to disperse corrosion to an even film, enabling the iron to resist pitting. Another study has shown that slag inclusions are pathways to corrosion. Other studies show that sulfur in the wrought iron decreases corrosion resistance, while phosphorus increases corrosion resistance. Chloride ions also decrease wrought iron's corrosion resistance.
Wrought iron may be welded in the same manner as mild steel, but the presence of oxide or inclusions will give defective results.
The material has a rough surface, so it can hold platings and coatings better. For instance, a galvanic zinc finish applied to wrought iron is approximately 25–40% thicker than the same finish on steel. In Table 1, the chemical composition of wrought iron is compared to that of pig iron and
slag
Slag is a by-product of smelting (pyrometallurgical) ores and used metals. Broadly, it can be classified as ferrous (by-products of processing iron and steel), ferroalloy (by-product of ferroalloy production) or non-ferrous/base metals (by-prod ...
inclusions (up to 2% by weight), which give it a wood-like "grain" that is visible when it is etched, rusted, or bent to failure
Failure is the state or condition of not meeting a desirable or intended objective (goal), objective, and may be viewed as the opposite of Success (concept), success. The criteria for failure depends on context, and may be relative to a parti ...
. Wrought iron is tough, malleable, ductile, corrosion resistant, and easily forge welded, but is more difficult to weld electrically.
Before the development of effective methods of steelmaking and the availability of large quantities of steel, wrought iron was the most common form of malleable iron. It was given the name ''wrought'' because it was hammered, rolled, or otherwise worked while hot enough to expel molten slag. The modern functional equivalent of wrought iron is mild steel
Carbon steel is a steel with carbon content from about 0.05 up to 2.1 percent by weight. The definition of carbon steel from the American Iron and Steel Institute (AISI) states:
* no minimum content is specified or required for chromium, cobalt ...
, also called low-carbon steel. Neither wrought iron nor mild steel contain enough carbon to be hardenable by heating and quenching.
Wrought iron is highly refined, with a small amount of silicate slag forged out into fibres. It comprises around 99.4% iron by mass. The presence of slag can be beneficial for blacksmithing operations, such as forge welding, since the silicate inclusions act as a flux
Flux describes any effect that appears to pass or travel (whether it actually moves or not) through a surface or substance. Flux is a concept in applied mathematics and vector calculus which has many applications to physics. For transport ph ...
and give the material its unique, fibrous structure. The silicate filaments in the slag also protect the iron from corrosion and diminish the effect of fatigue caused by shock and vibration.
Historically, a modest amount of wrought iron was refined into steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistant ty ...
, which was used mainly to produce sword
A sword is an edged, bladed weapon intended for manual cutting or thrusting. Its blade, longer than a knife or dagger, is attached to a hilt and can be straight or curved. A thrusting sword tends to have a straighter blade with a pointed ti ...
s, cutlery
Cutlery (also referred to as silverware, flatware, or tableware), includes any hand implement used in preparing, serving, and especially eating food in Western culture. A person who makes or sells cutlery is called a cutler. The city of Sheffie ...
, chisel
A chisel is a tool with a characteristically shaped cutting edge (such that wood chisels have lent part of their name to a particular grind) of blade on its end, for carving or cutting a hard material such as wood, stone, or metal by hand, stru ...
s, axes, and other edged tools, as well as springs and files. The demand for wrought iron reached its peak in the 1860s, being in high demand for ironclad warships and railway use. However, as properties such as brittleness of mild steel
Carbon steel is a steel with carbon content from about 0.05 up to 2.1 percent by weight. The definition of carbon steel from the American Iron and Steel Institute (AISI) states:
* no minimum content is specified or required for chromium, cobalt ...
improved with better ferrous metallurgy and as steel became less costly to make thanks to the Bessemer process and the Siemens-Martin process
An open-hearth furnace or open hearth furnace is any of several kinds of industrial furnace in which excess carbon and other impurities are burnt out of pig iron to produce steel. Because steel is difficult to manufacture owing to its high melt ...
, the use of wrought iron declined.
Many items, before they came to be made of mild steel
Carbon steel is a steel with carbon content from about 0.05 up to 2.1 percent by weight. The definition of carbon steel from the American Iron and Steel Institute (AISI) states:
* no minimum content is specified or required for chromium, cobalt ...
, were produced from wrought iron, including rivets, nails, wire, chains, rails, railway coupling
A coupling (or a coupler) is a mechanism typically placed at each end of a railway vehicle that connects them together to form a train. A variety of coupler types have been developed over the course of railway history. Key issues in their desig ...
s, water and steam pipes, nuts
Nut often refers to:
* Nut (fruit), fruit composed of a hard shell and a seed, or a collective noun for dry and edible fruits or seeds
* Nut (hardware), fastener used with a bolt
Nut or Nuts may also refer to:
Arts, entertainment, and media Com ...
, bolts, horseshoe
A horseshoe is a fabricated product designed to protect a horse hoof from wear. Shoes are attached on the palmar surface (ground side) of the hooves, usually nailed through the insensitive hoof wall that is anatomically akin to the human toen ...
s, handrails, wagon tires, straps for timber roof trusses, and ornamental ironwork
Ironwork is any weapon, artwork, utensil, or architectural feature made of iron, especially one used for decoration. There are two main types of ironwork: wrought iron and cast iron. While the use of iron dates as far back as 4000BC, it was th ...
, among many other things.
Wrought iron is no longer produced on a commercial scale. Many products described as wrought iron, such as guard rail
Guard rail, guardrails, or protective guarding, in general, are a boundary feature and may be a means to prevent or deter access to dangerous or off-limits areas while allowing light and visibility in a greater way than a fence. Common shapes ...
s, garden furniture, and gate
A gate or gateway is a point of entry to or from a space enclosed by walls. The word derived from old Norse "gat" meaning road or path; But other terms include ''yett and port''. The concept originally referred to the gap or hole in the wall ...
s are made of mild steel. They retain that description, because they are made to resemble objects which in the past were wrought (worked) by hand by a blacksmith (although many decorative iron objects, including fences and gates, were often cast rather than wrought).
Terminology
The word "wrought" is an archaic past participle of the verb "to work," and so "wrought iron" literally means "worked iron". Wrought iron is a general term for the commodity, but is also used more specifically for finished iron goods, as manufactured by a blacksmith. It was used in that narrower sense in BritishCustoms
Customs is an authority or agency in a country responsible for collecting tariffs and for controlling the flow of goods, including animals, transports, personal effects, and hazardous items, into and out of a country. Traditionally, customs ...
records, such manufactured iron was subject to a higher rate of duty than what might be called "unwrought" iron. Cast iron, unlike wrought iron, is brittle and cannot be worked either hot or cold. Cast iron can break if struck with a hammer.
In the 17th, 18th, and 19th centuries, wrought iron went by a wide variety of terms according to its form, origin, or quality.
While the bloomery
A bloomery is a type of metallurgical furnace once used widely for smelting iron from its oxides. The bloomery was the earliest form of smelter capable of smelting iron. Bloomeries produce a porous mass of iron and slag called a ''bloom ...
process produced wrought iron directly from ore, cast iron or pig iron
Pig iron, also known as crude iron, is an intermediate product of the iron industry in the production of steel which is obtained by smelting iron ore in a blast furnace. Pig iron has a high carbon content, typically 3.8–4.7%, along with silic ...
were the starting materials used in the finery forge and puddling furnace. Pig iron and cast iron have higher carbon content than wrought iron, but have a lower melting point than iron or steel. Cast and especially pig iron have excess slag which must be at least partially removed to produce quality wrought iron. At foundries it was common to blend scrap wrought iron with cast iron to improve the physical properties of castings.
For several years after the introduction of Bessemer and open hearth steel, there were different opinions as to what differentiated iron from steel; some believed it was the chemical composition and others that it was whether the iron heated sufficiently to melt and "fuse". Fusion eventually became generally accepted as relatively more important than composition below a given low carbon concentration. Another difference is that steel can be hardened by heat treating.
Historically, wrought iron was known as "commercially pure iron", however, it no longer qualifies because current standards for commercially pure iron require a carbon content of less than 0.008 wt%.
Types and shapes
Bar iron is a generic term sometimes used to distinguish it from cast iron. It is the equivalent of an ingot of cast metal, in a convenient form for handling, storage, shipping and further working into a finished product. The bars were the usual product of the finery forge, but not necessarily made by that process. * Rod iron—cut from flat bar iron in a slitting mill provided the raw material for spikes and nails. * Hoop iron—suitable for the hoops of barrels, made by passing rod iron through rolling dies. * Plate iron—sheets suitable for use as boiler plate. *Blackplate
Blackplate is hot rolled or cold rolled,DIN 55405:2006-11 ''Verpackung - Terminologie - Begriffe'', Berlin: Beuth Verlag. non-descaled sheet steel or sheet iron.
—sheets, perhaps thinner than plate iron, from the black rolling stage of tinplate production.
* Voyage iron—narrow flat bar iron, made or cut into bars of a particular weight, a commodity for sale in Africa for the Atlantic slave trade
The Atlantic slave trade, transatlantic slave trade, or Euro-American slave trade involved the transportation by slave traders of enslaved African people, mainly to the Americas. The slave trade regularly used the triangular trade route and i ...
. The number of bars per ton gradually increased from 70 per ton in the 1660s to 75–80 per ton in 1685 and "near 92 to the ton" in 1731.
Origin
* Charcoal iron—until the end of the 18th century, wrought iron was smelted from ore using charcoal, by thebloomery
A bloomery is a type of metallurgical furnace once used widely for smelting iron from its oxides. The bloomery was the earliest form of smelter capable of smelting iron. Bloomeries produce a porous mass of iron and slag called a ''bloom ...
process. Wrought iron was also produced from pig iron
Pig iron, also known as crude iron, is an intermediate product of the iron industry in the production of steel which is obtained by smelting iron ore in a blast furnace. Pig iron has a high carbon content, typically 3.8–4.7%, along with silic ...
using a finery forge or in a Lancashire hearth. The resulting metal was highly variable, both in chemistry and slag content.
* Puddled iron—the puddling process was the first large-scale process to produce wrought iron. In the puddling process, pig iron is refined in a reverberatory furnace to prevent contamination of the iron from the sulfur in the coal or coke. The molten pig iron is manually stirred, exposing the iron to atmospheric oxygen, which decarburizes the iron. As the iron is stirred, globs of wrought iron are collected into balls by the stirring rod (rabble arm or rod) and those are periodically removed by the puddler. Puddling was patented in 1784 and became widely used after 1800. By 1876, annual production of puddled iron in the UK alone was over 4 million tons. Around that time, the open hearth furnace was able to produce steel of suitable quality for structural purposes, and wrought iron production went into decline.
* Oregrounds iron—a particularly pure grade of bar iron made ultimately from iron ore
Iron ores are rocks and minerals from which metallic iron can be economically extracted. The ores are usually rich in iron oxides and vary in color from dark grey, bright yellow, or deep purple to rusty red. The iron is usually found in the fo ...
from the Dannemora mine in Sweden
Sweden, formally the Kingdom of Sweden,The United Nations Group of Experts on Geographical Names states that the country's formal name is the Kingdom of SwedenUNGEGN World Geographical Names, Sweden./ref> is a Nordic country located on ...
. Its most important use was as the raw material for the cementation process
The cementation process is an obsolete technology for making steel by carburization of iron. Unlike modern steelmaking, it increased the amount of carbon in the iron. It was apparently developed before the 17th century. Derwentcote Steel F ...
of steelmaking.
* Danks iron—originally iron imported to Great Britain from Gdańsk
Gdańsk ( , also ; ; csb, Gduńsk;Stefan Ramułt, ''Słownik języka pomorskiego, czyli kaszubskiego'', Kraków 1893, Gdańsk 2003, ISBN 83-87408-64-6. , Johann Georg Theodor Grässe, ''Orbis latinus oder Verzeichniss der lateinischen Benen ...
, but in the 18th century more probably the kind of iron (from eastern Sweden) that once came from Gdańsk.
* Forest iron—iron from the English Forest of Dean, where haematite
Hematite (), also spelled as haematite, is a common iron oxide compound with the formula, Fe2O3 and is widely found in rocks and soils. Hematite crystals belong to the rhombohedral lattice system which is designated the alpha polymorph of . ...
ore enabled tough iron to be produced.
* Lukes iron—iron imported from Liège
Liège ( , , ; wa, Lîdje ; nl, Luik ; german: Lüttich ) is a major city and municipality of Wallonia and the capital of the Belgian province of Liège.
The city is situated in the valley of the Meuse, in the east of Belgium, not far from b ...
, whose Dutch name is "Luik."
* Ames iron or amys iron—another variety of iron imported to England from northern Europe. Its origin has been suggested to be Amiens, but it seems to have been imported from Flanders in the 15th century and Holland later, suggesting an origin in the Rhine valley. Its origins remain controversial.
* Botolf iron or Boutall iron—from Bytów (Polish Pomerania) or Bytom
Bytom (Polish pronunciation: ; Silesian: ''Bytōm, Bytōń'', german: Beuthen O.S.) is a city in Upper Silesia, in southern Poland. Located in the Silesian Voivodeship of Poland, the city is 7 km northwest of Katowice, the regional capital ...
(Polish Silesia).
* Sable iron (or Old Sable)—iron bearing the mark (a sable
The sable (''Martes zibellina'') is a species of marten, a small omnivorous mammal primarily inhabiting the forest environments of Russia, from the Ural Mountains throughout Siberia, and northern Mongolia. Its habitat also borders eastern Kaza ...
) of the Demidov family of Russian ironmasters, one of the better brands of Russian iron
Russia iron or Russian iron refers to a type of sheet iron produced in Russia during the 19th and early 20th century.
This iron sheeting had a smooth, glossy black surface coating, sometimes greenish-tinged, which did not flake upon bending and mad ...
.
Quality
;Tough iron: Also spelled "tuf", is not brittle and is strong enough to be used for tools. ;Blend iron: Made using a mixture of different types ofpig iron
Pig iron, also known as crude iron, is an intermediate product of the iron industry in the production of steel which is obtained by smelting iron ore in a blast furnace. Pig iron has a high carbon content, typically 3.8–4.7%, along with silic ...
.
;Best iron: Iron put through several stages of piling and rolling to reach the stage regarded (in the 19th century) as the best quality.
;Marked bar iron: Made by members of the Marked Bar Association
In linguistics and social sciences, markedness is the state of standing out as nontypical or divergent as opposed to regular or common. In a marked–unmarked relation, one term of an opposition is the broader, dominant one. The dominant defau ...
and marked with the maker's brand mark as a sign of its quality.
Defects
Wrought iron is a form of commercial iron containing less than 0.10% of carbon, less than 0.25% of impurities total of sulfur, phosphorus, silicon and manganese, and less than 2% slag by weight. Wrought iron is '' redshort'' or ''hot short'' if it contains sulfur in excess quantity. It has sufficient tenacity when cold, but cracks when bent or finished at a red heat. Hot short iron was considered unmarketable. ''Cold short'' iron, also known as ''coldshear'', ''colshire'', contains excessive phosphorus. It is very brittle when cold and cracks if bent. It may, however, be worked at high temperature. Historically, coldshort iron was considered sufficient for nails. Phosphorus is not necessarily detrimental to iron. Ancient Near Eastern smiths did not add lime to their furnaces. The absence of calcium oxide in the slag, and the deliberate use of wood with high phosphorus content during the smelting, induces a higher phosphorus content (typically <.3%) than in modern iron (<.02-.03%). Analysis of the Iron Pillar of Delhi gives 0.11% in the iron. The included slag in wrought iron also imparts corrosion resistance. The presence of phosphorus (without carbon) produces a ductile iron suitable for wire drawing for piano wire.History
Western world
 Wrought iron has been used for many centuries, and is the "iron" that is referred to throughout Western history. The other form of iron, cast iron, was in use in China since ancient times but was not introduced into Western Europe until the 15th century; even then, due to its brittleness, it could be used for only a limited number of purposes. Throughout much of the Middle Ages, iron was produced by the direct reduction of ore in manually operated
Wrought iron has been used for many centuries, and is the "iron" that is referred to throughout Western history. The other form of iron, cast iron, was in use in China since ancient times but was not introduced into Western Europe until the 15th century; even then, due to its brittleness, it could be used for only a limited number of purposes. Throughout much of the Middle Ages, iron was produced by the direct reduction of ore in manually operated bloomeries
A bloomery is a type of metallurgical furnace once used widely for smelting iron from its oxides. The bloomery was the earliest form of smelter capable of smelting iron. Bloomeries produce a porous mass of iron and slag called a ''bloom''. ...
, although water power had begun to be employed by 1104.
The raw material produced by all indirect processes is pig iron. It has a high carbon content and as a consequence, it is brittle and cannot be used to make hardware. The osmond process was the first of the indirect processes, developed by 1203, but bloomery production continued in many places. The process depended on the development of the blast furnace, of which medieval examples have been discovered at Lapphyttan, Sweden and in Germany.
The bloomery and osmond processes were gradually replaced from the 15th century by finery processes, of which there were two versions, the German and Walloon. They were in turn replaced from the late 18th century by puddling
A puddle is a small accumulation of liquid on a surface.
Puddle or Puddles may also refer to:
* Puddle, Cornwall, hamlet in England
* ''Puddle'' (video game)
* Puddle (M. C. Escher), a woodcut by M. C. Escher
* Weld puddle, a crucial part of the ...
, with certain variants such as the Swedish Lancashire process
The Lancashire hearth was used to fine pig iron, removing carbon to produce wrought iron.
Origins
Until the early 19th century, the usual method of producing wrought iron involved a charcoal-fired finery in a finery forge. In the beginning of t ...
. Those, too, are now obsolete, and wrought iron is no longer manufactured commercially.
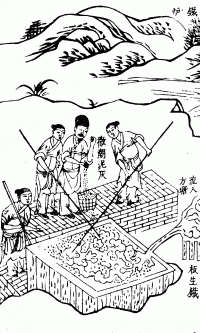
China
During the Han dynasty (202 BC – 220 AD), new iron smelting processes led to the manufacture of new wrought iron implements for use in agriculture, such as the multi-tube seed drill andiron plough
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in front ...
. In addition to accidental lumps of low-carbon wrought iron produced by excessive injected air in ancient Chinese cupola furnaces. The ancient Chinese created wrought iron by using the finery forge at least by the 2nd century BC, the earliest specimens of cast and pig iron
Pig iron, also known as crude iron, is an intermediate product of the iron industry in the production of steel which is obtained by smelting iron ore in a blast furnace. Pig iron has a high carbon content, typically 3.8–4.7%, along with silic ...
fined into wrought iron and steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistant ty ...
found at the early Han Dynasty site at Tieshengguo. Pigott speculates that the finery forge existed in the previous Warring States period (403–221 BC), due to the fact that there are wrought iron items from China dating to that period and there is no documented evidence of the bloomery
A bloomery is a type of metallurgical furnace once used widely for smelting iron from its oxides. The bloomery was the earliest form of smelter capable of smelting iron. Bloomeries produce a porous mass of iron and slag called a ''bloom ...
ever being used in China. The fining process involved liquifying cast iron in a fining hearth and removing carbon from the molten cast iron through oxidation. Wagner writes that in addition to the Han Dynasty hearths believed to be fining hearths, there is also pictorial evidence of the fining hearth from a Shandong
Shandong ( , ; ; alternately romanized as Shantung) is a coastal province of the People's Republic of China and is part of the East China region.
Shandong has played a major role in Chinese history since the beginning of Chinese civilizati ...
tomb mural dated 1st to 2nd century AD, as well as a hint of written evidence in the 4th century AD Daoist text '' Taiping Jing''.
Bloomery process
Wrought iron was originally produced by a variety of smelting processes, all described today as "bloomeries". Different forms of bloomery were used at different places and times. The bloomery was charged withcharcoal
Charcoal is a lightweight black carbon residue produced by strongly heating wood (or other animal and plant materials) in minimal oxygen to remove all water and volatile constituents. In the traditional version of this pyrolysis process, cal ...
and iron ore and then lit. Air was blown in through a tuyere to heat the bloomery to a temperature somewhat below the melting point of iron. In the course of the smelt, slag would melt and run out, and carbon monoxide from the charcoal would reduce the ore to iron, which formed a spongy mass (called a "bloom") containing iron and also molten silicate minerals (slag) from the ore. The iron remained in the solid state. If the bloomery were allowed to become hot enough to melt the iron, carbon would dissolve into it and form pig or cast iron, but that was not the intention. However, the design of a bloomery made it difficult to reach the melting point of iron and also prevented the concentration of carbon monoxide from becoming high.
After smelting was complete, the bloom was removed, and the process could then be started again. It was thus a batch process, rather than a continuous one such as a blast furnace. The bloom had to be forged mechanically to consolidate it and shape it into a bar, expelling slag in the process.
During the Middle Ages, water-power was applied to the process, probably initially for powering bellows, and only later to hammers for forging the blooms. However, while it is certain that water-power was used, the details remain uncertain. That was the culmination of the direct process of ironmaking. It survived in Spain and southern France as Catalan Forges to the mid 19th century, in Austria as the ''stuckofen'' to 1775, and near Garstang
Garstang is an ancient market town and civil parish within the Wyre borough of Lancashire, England. It is north of the city of Preston and the same distance south of Lancaster.
In 2011, the parish had a total resident population of 4,268 ...
in England until about 1770; it was still in use with hot blast in New York
New York most commonly refers to:
* New York City, the most populous city in the United States, located in the state of New York
* New York (state), a state in the northeastern United States
New York may also refer to:
Film and television
* '' ...
in the 1880s. In Japan
Japan ( ja, 日本, or , and formally , ''Nihonkoku'') is an island country in East Asia. It is situated in the northwest Pacific Ocean, and is bordered on the west by the Sea of Japan, while extending from the Sea of Okhotsk in the north ...
the last of the old '' tatara'' bloomeries used in production of traditional tamahagane steel, mainly used in swordmaking, was extinguished only in 1925, though in the late 20th century the production resumed on a low scale to supply the steel to the artisan swordmakers.
Osmond process
Osmond iron consisted of balls of wrought iron, produced by melting pig iron and catching the droplets on a staff, which was spun in front of a blast of air so as to expose as much of it as possible to the air and oxidise its carbon content. The resultant ball was often forged into bar iron in a hammer mill.Finery process
In the 15th century, theblast furnace
A blast furnace is a type of metallurgical furnace used for smelting to produce industrial metals, generally pig iron, but also others such as lead or copper. ''Blast'' refers to the combustion air being "forced" or supplied above atmospheric ...
spread into what is now Belgium where it was improved. From there, it spread via the Pays de Bray on the boundary of Normandy and then to the Weald in England. With it, the finery forge spread. Those remelted the pig iron and (in effect) burnt out the carbon, producing a bloom, which was then forged into bar iron. If rod iron was required, a slitting mill was used.
The finery process existed in two slightly different forms. In Great Britain, France, and parts of Sweden, only the Walloon process was used. That employed two different hearths, a finery hearth for finishing the iron and a chafery hearth for reheating it in the course of drawing the bloom out into a bar. The finery always burnt charcoal, but the chafery could be fired with mineral coal, since its impurities would not harm the iron when it was in the solid state. On the other hand, the German process, used in Germany, Russia, and most of Sweden used a single hearth for all stages.
The introduction of coke for use in the blast furnace by Abraham Darby in 1709 (or perhaps others a little earlier) initially had little effect on wrought iron production. Only in the 1750s was coke pig iron used on any significant scale as the feedstock of finery forges. However, charcoal continued to be the fuel for the finery.
Potting and stamping
From the late 1750s, ironmasters began to develop processes for making bar iron without charcoal. There were a number of patented processes for that, which are referred to today as potting and stamping. The earliest were developed by John Wood of Wednesbury and his brother Charles Wood of Low Mill at Egremont, patented in 1763. Another was developed for the Coalbrookdale Company by theCranage brothers
Thomas and George Cranege (also spelled ''Cranage''), who worked in the ironworking industry in England in the 1760s, are notable for introducing a new method of producing wrought iron from pig iron.
Experiment of 1766
The process of converting p ...
. Another important one was that of John Wright and Joseph Jesson of West Bromwich.
Puddling process
 A number of processes for making wrought iron without charcoal were devised as the Industrial Revolution began during the latter half of the 18th century. The most successful of those was puddling, using a puddling furnace (a variety of the reverberatory furnace), which was invented by Henry Cort in 1784. It was later improved by others including Joseph Hall, who was the first to add iron oxide to the charge. In that type of furnace, the metal does not come into contact with the fuel, and so is not contaminated by its impurities . The heat of the combustion products pass over the surface of the puddle and the roof of the furnace reverberates (reflects) the heat onto the metal puddle on the fire bridge of the furnace.
Unless the raw material used is white cast iron, the pig iron or other raw product of the puddling first had to be refined into
A number of processes for making wrought iron without charcoal were devised as the Industrial Revolution began during the latter half of the 18th century. The most successful of those was puddling, using a puddling furnace (a variety of the reverberatory furnace), which was invented by Henry Cort in 1784. It was later improved by others including Joseph Hall, who was the first to add iron oxide to the charge. In that type of furnace, the metal does not come into contact with the fuel, and so is not contaminated by its impurities . The heat of the combustion products pass over the surface of the puddle and the roof of the furnace reverberates (reflects) the heat onto the metal puddle on the fire bridge of the furnace.
Unless the raw material used is white cast iron, the pig iron or other raw product of the puddling first had to be refined into refined iron
Pig iron, also known as crude iron, is an intermediate product of the iron industry in the production of steel which is obtained by smelting iron ore in a blast furnace. Pig iron has a high carbon content, typically 3.8–4.7%, along with s ...
, or finers metal. That would be done in a refinery where raw coal was used to remove silicon and convert carbon within the raw material, found in the form of graphite, to a combination with iron called cementite.
In the fully developed process (of Hall), this metal was placed into the hearth of the puddling furnace where it was melted. The hearth was lined with oxidizing agents such as haematite
Hematite (), also spelled as haematite, is a common iron oxide compound with the formula, Fe2O3 and is widely found in rocks and soils. Hematite crystals belong to the rhombohedral lattice system which is designated the alpha polymorph of . ...
and iron oxide. The mixture was subjected to a strong current of air and stirred with long bars, called puddling bars or rabbles, through working doors. The air, the stirring, and the "boiling" action of the metal helped the oxidizing agents to oxidize the impurities and carbon out of the pig iron. As the impurities oxidize, they formed a molten slag or drifted off as gas, while the remaining iron solidified into spongy wrought iron that floated to the top of the puddle and was fished out of the melt as puddle balls, using puddle bars.
Shingling
There was still some slag left in the puddle balls, so while they were still hot they would be shingled to remove the remaining slag and cinder. That was achieved by forging the balls under a hammer, or by squeezing the bloom in a machine. The material obtained at the end of shingling is known as bloom. The blooms are not useful in that form, so they were rolled into a final product. Sometimes European ironworks would skip the shingling process completely and roll the puddle balls. The only drawback to that is that the edges of the rough bars were not as well compressed. When the rough bar was reheated, the edges might separate and be lost into the furnace.Rolling
The bloom was passed through rollers and to produce bars. The bars of wrought iron were of poor quality, called muck bars or puddle bars. To improve their quality, the bars were cut up, piled and tied together by wires, a process known as faggoting or piling. They were then reheated to a welding state, forge welded, and rolled again into bars. The process could be repeated several times to produce wrought iron of desired quality. Wrought iron that has been rolled multiple times is called merchant bar or merchant iron.Lancashire process
The advantage of puddling was that it used coal, not charcoal as fuel. However, that was of little advantage in Sweden, which lacked coal.Gustaf Ekman
Gustav, Gustaf or Gustave may refer to:
*Gustav (name), a male given name of Old Swedish origin
Art, entertainment, and media
*Primeval (film), ''Primeval'' (film), a 2007 American horror film
*Gustav (film series), ''Gustav'' (film series), a Hu ...
observed charcoal fineries at Ulverston, which were quite different from any in Sweden. After his return to Sweden in the 1830s, he experimented and developed a process similar to puddling but used firewood and charcoal, which was widely adopted in the Bergslagen in the following decades.
Aston process
In 1925, James Aston of the United States developed a process for manufacturing wrought iron quickly and economically. It involved taking molten steel from a Bessemer converter and pouring it into cooler liquid slag. The temperature of the steel is about 1500 °C and the liquid slag is maintained at approximately 1200 °C. The molten steel contains a large amount of dissolved gases so when the liquid steel hit the cooler surfaces of the liquid slag the gases were liberated. The molten steel then froze to yield a spongy mass having a temperature of about 1370 °C. The spongy mass would then be finished by being shingled and rolled as described under puddling (above). Three to four tons could be converted per batch with the method.Decline
Steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistant ty ...
began to replace iron for railroad rails as soon as the Bessemer process for its manufacture was adopted (1865 on). Iron remained dominant for structural applications until the 1880s, because of problems with brittle steel, caused by introduced nitrogen, high carbon, excess phosphorus, or excessive temperature during or too-rapid rolling. By 1890 steel had largely replaced iron for structural applications.
Sheet iron (Armco 99.97% pure iron) had good properties for use in appliances, being well-suited for enamelling and welding, and being rust-resistant.
In the 1960s, the price of steel production was dropping due to recycling, and even using the Aston process, wrought iron production was labor-intensive. It has been estimated that the production of wrought iron is approximately twice as expensive as that of low-carbon steel. In the United States, the last plant closed in 1969. The last in the world was the Atlas Forge of Thomas Walmsley and Sons in Bolton, Great Britain, which closed in 1973. Its 1860s-era equipment was moved to the Blists Hill site of Ironbridge Gorge Museum for preservation. Some wrought iron is still being produced for heritage restoration purposes, but only by recycling scrap.
Properties
 The slag inclusions, or stringers, in wrought iron give it properties not found in other forms of ferrous metal. There are approximately 250,000 inclusions per square inch. A fresh fracture shows a clear bluish color with a high silky luster and fibrous appearance.
Wrought iron lacks the carbon content necessary for hardening through heat treatment, but in areas where steel was uncommon or unknown, tools were sometimes cold-worked (hence cold iron) to harden them. An advantage of its low carbon content is its excellent weldability. Furthermore, sheet wrought iron cannot bend as much as steel sheet metal when cold worked. Wrought iron can be melted and cast, however the product is no longer wrought iron, since the slag stringers characteristic of wrought iron disappear on melting, so the product resembles impure, cast, Bessemer steel. There is no engineering advantage as compared to cast iron or steel, both of which are cheaper.
Due to the variations in iron ore origin and iron manufacture, wrought iron can be inferior or superior in corrosion resistance, compared to other iron alloys. There are many mechanisms behind its corrosion resistance. Chilton and Evans found that nickel enrichment bands reduce corrosion. They also found that in puddled, forged, and piled iron, the working-over of the metal spread out copper, nickel, and tin impurities that produce electrochemical conditions that slow down corrosion. The slag inclusions have been shown to disperse corrosion to an even film, enabling the iron to resist pitting. Another study has shown that slag inclusions are pathways to corrosion. Other studies show that sulfur in the wrought iron decreases corrosion resistance, while phosphorus increases corrosion resistance. Chloride ions also decrease wrought iron's corrosion resistance.
Wrought iron may be welded in the same manner as mild steel, but the presence of oxide or inclusions will give defective results.
The material has a rough surface, so it can hold platings and coatings better. For instance, a galvanic zinc finish applied to wrought iron is approximately 25–40% thicker than the same finish on steel. In Table 1, the chemical composition of wrought iron is compared to that of pig iron and
The slag inclusions, or stringers, in wrought iron give it properties not found in other forms of ferrous metal. There are approximately 250,000 inclusions per square inch. A fresh fracture shows a clear bluish color with a high silky luster and fibrous appearance.
Wrought iron lacks the carbon content necessary for hardening through heat treatment, but in areas where steel was uncommon or unknown, tools were sometimes cold-worked (hence cold iron) to harden them. An advantage of its low carbon content is its excellent weldability. Furthermore, sheet wrought iron cannot bend as much as steel sheet metal when cold worked. Wrought iron can be melted and cast, however the product is no longer wrought iron, since the slag stringers characteristic of wrought iron disappear on melting, so the product resembles impure, cast, Bessemer steel. There is no engineering advantage as compared to cast iron or steel, both of which are cheaper.
Due to the variations in iron ore origin and iron manufacture, wrought iron can be inferior or superior in corrosion resistance, compared to other iron alloys. There are many mechanisms behind its corrosion resistance. Chilton and Evans found that nickel enrichment bands reduce corrosion. They also found that in puddled, forged, and piled iron, the working-over of the metal spread out copper, nickel, and tin impurities that produce electrochemical conditions that slow down corrosion. The slag inclusions have been shown to disperse corrosion to an even film, enabling the iron to resist pitting. Another study has shown that slag inclusions are pathways to corrosion. Other studies show that sulfur in the wrought iron decreases corrosion resistance, while phosphorus increases corrosion resistance. Chloride ions also decrease wrought iron's corrosion resistance.
Wrought iron may be welded in the same manner as mild steel, but the presence of oxide or inclusions will give defective results.
The material has a rough surface, so it can hold platings and coatings better. For instance, a galvanic zinc finish applied to wrought iron is approximately 25–40% thicker than the same finish on steel. In Table 1, the chemical composition of wrought iron is compared to that of pig iron and carbon steel
Carbon steel is a steel with carbon content from about 0.05 up to 2.1 percent by weight. The definition of carbon steel from the American Iron and Steel Institute (AISI) states:
* no minimum content is specified or required for chromium, cobalt ...
. Although it appears that wrought iron and plain carbon steel have similar chemical compositions, that is deceptive. Most of the manganese, sulfur, phosphorus, and silicon are incorporated into the slag fibers in the wrought iron, making wrought iron purer than plain carbon steel.
Amongst its other properties, wrought iron becomes soft at red heat and can be easily forged and forge welded. It can be used to form temporary magnets, but it cannot be magnetized permanently, and is ductile, malleable, and tough
Tough may refer to:
* Toughness, the resistance to fracture of a material when stressed
* Machismo, prominently exhibited or excessive masculinity
* Psychological resilience
Tough may also refer to:
People
* Allen Tough (1936–2012), Canadian ...
.
Ductility
For most purposes, ductility rather than tensile strength is a more important measure of the quality of wrought iron. In tensile testing, the best irons are able to undergo considerable elongation before failure. Higher tensile wrought iron is brittle. Because of the large number of boiler explosions on steamboats, the U.S. Congress passed legislation in 1830 which approved funds for correcting the problem. The treasury awarded a $1500 contract to the Franklin Institute to conduct a study. As part of the study, Walter R. Johnson and Benjamin Reeves conducted strength tests on boiler iron using a tester they had built in 1832 based on the design of Lagerhjelm in Sweden. Unfortunately, because of the misunderstanding of tensile strength and ductility, their work did little to reduce failures. The importance of ductility was recognized by some very early in the development of tube boilers, evidenced by Thurston's comment: Various 19th century investigations of boiler explosions, especially those by insurance companies, found causes to be most commonly the result of operating boilers above the safe pressure range, either to get more power, or due to defective boiler pressure relief valves and difficulties of obtaining reliable indications of pressure and water levels. Poor fabrication was also a common problem. Also, the thickness of the iron in steam drums was low, by modern standards. By the late 19th century, when metallurgists were able to better understand what properties and processes made good iron, it was being displaced by steel. Also, the old cylindrical boilers with fire tubes were displaced by water tube boilers, which are inherently safer.Purity
In 2010, Dr. Gerry McDonnell demonstrated in England by analysis that a wrought iron bloom, from a traditional smelt, could be worked into 99.7% pure iron with no evidence of carbon. It was found that the stringers common to other wrought irons were not present, thus making it very malleable for the smith to work hot and cold. A commercial source of pure iron is available and is used by smiths as an alternative to traditional wrought iron and other new generation ferrous metals.Applications
Wrought iron furniture has a long history, dating back to Roman times. There are 13th century wrought iron gates in Westminster Abbey in London, and wrought iron furniture seemed to reach its peak popularity in Britain in the 17th century, during the reign ofWilliam III William III or William the Third may refer to:
Kings
* William III of Sicily (c. 1186–c. 1198)
* William III of England and Ireland or William III of Orange or William II of Scotland (1650–1702)
* William III of the Netherlands and Luxembourg ...
and Mary II
Mary II (30 April 166228 December 1694) was Queen of England, Scotland, and Ireland, co-reigning with her husband, William III & II, from 1689 until her death in 1694.
Mary was the eldest daughter of James, Duke of York, and his first wife ...
. However, cast iron and cheaper steel caused a gradual decline in wrought iron manufacture; the last wrought ironworks in Britain closed in 1974.
It is also used to make home decor items such as baker's racks, wine racks, pot rack
A pot rack is a functional piece of kitchen furniture that is used to hang or store cooking pot
Cookware and bakeware is food preparation equipment, such as cooking pots, pans, baking sheets etc. used in kitchens. Cookware is used on a stov ...
s, etageres, table bases, desks, gates, beds, candle holders, curtain rods, bars, and bar stools.
The vast majority of wrought iron available today is from reclaimed materials. Old bridges and anchor chains dredged from harbors are major sources. The greater corrosion resistance of wrought iron is due to the siliceous impurities (naturally occurring in iron ore), namely ferric silicate
In chemistry, a silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is al ...
.
Wrought iron has been used for decades as a generic term across the gate and fencing industry, even though mild steel
Carbon steel is a steel with carbon content from about 0.05 up to 2.1 percent by weight. The definition of carbon steel from the American Iron and Steel Institute (AISI) states:
* no minimum content is specified or required for chromium, cobalt ...
is used for manufacturing these "wrought iron" gates. This is mainly because of the limited availability of true wrought iron. Steel can also be hot-dip galvanised to prevent corrosion, which cannot be done with wrought iron.
See also
* Bronze and brass ornamental work * Cast iron * Semi-steel castingNotes
References
Further reading
* *External links
* {{Authority control Architectural elements Building materials Chinese inventions Ferrous alloys Han dynasty Iron Ironmongery Metalworking