Umpolung on:
[Wikipedia]
[Google]
[Amazon]
In
 The net result of the benzoin reaction is that a bond has been formed between two carbons that are normally electrophiles.
The net result of the benzoin reaction is that a bond has been formed between two carbons that are normally electrophiles.
 Enzymes which use TPP as a cofactor can catalyze umpolung reactivity, such as the decarboxylation of pyruvate.
Enzymes which use TPP as a cofactor can catalyze umpolung reactivity, such as the decarboxylation of pyruvate.
 In the absence of TPP, the decarboxylation of pyruvate would result in the placement of a negative charge on the carbonyl carbon, which would run counter to the normal polarization of the carbon-oxygen double bond.
In the absence of TPP, the decarboxylation of pyruvate would result in the placement of a negative charge on the carbonyl carbon, which would run counter to the normal polarization of the carbon-oxygen double bond.
 3-membered rings are strained moieties in organic chemistry. When a 3-membered ring contains a heteroatom, such as in an
3-membered rings are strained moieties in organic chemistry. When a 3-membered ring contains a heteroatom, such as in an
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; ...
, umpolung () or polarity inversion is the chemical modification of a functional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest ...
with the aim of the reversal of polarity
Polarity may refer to:
Science
*Electrical polarity, direction of electrical current
*Polarity (mutual inductance), the relationship between components such as transformer windings
* Polarity (projective geometry), in mathematics, a duality of ord ...
of that group. This modification allows secondary reactions of this functional group that would otherwise not be possible. The concept was introduced by D. Seebach (hence the German word for reversed polarity) and E.J. Corey
Elias James Corey (born July 12, 1928) is an American organic chemist. In 1990, he won the Nobel Prize in Chemistry "for his development of the theory and methodology of organic synthesis", specifically retrosynthetic analysis. Regarded by many ...
. Polarity analysis during retrosynthetic analysis
Retrosynthetic analysis is a technique for solving problems in the planning of organic syntheses. This is achieved by transforming a target molecule into simpler precursor structures regardless of any potential reactivity/interaction with reagents. ...
tells a chemist when umpolung tactics are required to synthesize a target molecule.
Introduction
The vast majority of important organic molecules contain heteroatoms, which polarize carbon skeletons by virtue of their electronegativity. Therefore, in standard organic reactions, the majority of new bonds are formed between atoms of opposite polarity. This can be considered to be the "normal" mode of reactivity. One consequence of this natural polarization of molecules is that 1,3- and 1,5- heteroatom substituted carbon skeletons are extremely easy to synthesize (Aldol reaction
The aldol reaction is a means of forming carbon–carbon bonds in organic chemistry.
Discovered independently by the Russian chemist Alexander Borodin in 1869 and by the French chemist Charles-Adolphe Wurtz in 1872, the reaction combines two carb ...
, Claisen condensation
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone. It is named after Ra ...
, Michael reaction
In organic chemistry, the Michael reaction or Michael addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a carbon ...
, Claisen rearrangement
The Claisen rearrangement is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen. The heating of an allyl vinyl ether will initiate a ,3sigmatropic rearrangement to give a γ,δ-unsaturated carbonyl, ...
, Diels-Alder reaction), whereas 1,2-, 1,4-, and 1,6- heteroatom substitution patterns are more difficult to access via "normal" reactivity. It is therefore important to understand and develop methods to induce umpolung in organic reactions.
Examples
The simplest method of obtaining 1,2-, 1,4-, and 1,6- heteroatom substitution patterns is to start with them. Biochemical and industrial processes can provide inexpensive sources of chemicals that have normally inaccessible substitution patterns. For example, amino acids, oxalic acid, succinic acid, adipic acid, tartaric acid, and glucose are abundant and provide nonroutine substitution patterns.Cyanide-type umpolung
The canonical umpolung reagent is the cyanide ion. The cyanide ion is unusual in that a carbon triply bonded to a nitrogen would be expected to have a (+) polarity due to the higher electronegativity of the nitrogen atom. Yet, the negative charge of the cyanide ion is localized on the carbon, giving it a (-) formal charge. This chemical ambivalence results in umpolung in many reactions where cyanide is involved. For example, cyanide is a key catalyst in thebenzoin condensation
The benzoin addition is an addition reaction involving two aldehydes. The reaction generally occurs between aromatic aldehydes or glyoxals, and results in formation of an acyloin
Acyloins or α-hydroxy ketones are a class of organic compounds w ...
, a classical example of polarity inversion.
 The net result of the benzoin reaction is that a bond has been formed between two carbons that are normally electrophiles.
The net result of the benzoin reaction is that a bond has been formed between two carbons that are normally electrophiles.
N-heterocyclic carbenes
N-heterocyclic carbenes
A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for ex ...
, are similar to cyanide in reactivity. Like cyanide, they have an unusual chemical ambivalence, which allows it to trigger umpolung in reactions where it is involved. The carbene has six electrons - two each in the carbon-nitrogen single bonds, two in its sp2-hybridized orbital, and an empty p-orbital. The sp2 lone pair acts as an electron donor, whereas the empty p-orbital is capable as acting as an electron acceptor.
In this example, the β-carbon of the α,β-unsaturated ester 1 formally acts as a nucleophile, whereas normally it would be expected to be a Michael acceptor
In organic chemistry, the Michael reaction or Michael addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a carbon ...
.
This carbene reacts with the α,β-unsaturated ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
1 at the β-position forming the intermediate enolate 2. Through tautomerization
Tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert.
The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydr ...
2b can displace the terminal bromine atom to 3. An elimination reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 ...
regenerates the carbene and releases the product 4.
For comparison: in the Baylis-Hillman reaction the same electrophilic β-carbon atom is attacked by a reagent but resulting in the activation of the α-position of the enone as the nucleophile.
Thiamine pyrophosphate
Biological processes can employ cyanide-like umpolung reactivity without having to rely on the toxic cyanide ion.Thiamine
Thiamine, also known as thiamin and vitamin B1, is a vitamin, an essential micronutrient, that cannot be made in the body. It is found in food and commercially synthesized to be a dietary supplement or medication. Phosphorylated forms of thi ...
(which itself is an N-heterocyclic carbene
A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for ex ...
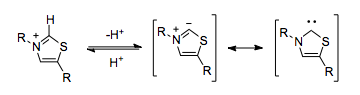
) pyrophosphate (TPP) serves a functionally identical role. The thiazolium ring in TPP is deprotonated within the hydrophobic core of the enzyme, resulting in a carbene which is capable of umpolung.  Enzymes which use TPP as a cofactor can catalyze umpolung reactivity, such as the decarboxylation of pyruvate.
Enzymes which use TPP as a cofactor can catalyze umpolung reactivity, such as the decarboxylation of pyruvate.
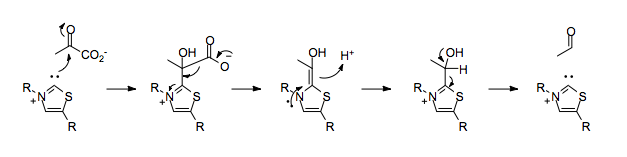
 In the absence of TPP, the decarboxylation of pyruvate would result in the placement of a negative charge on the carbonyl carbon, which would run counter to the normal polarization of the carbon-oxygen double bond.
In the absence of TPP, the decarboxylation of pyruvate would result in the placement of a negative charge on the carbonyl carbon, which would run counter to the normal polarization of the carbon-oxygen double bond.
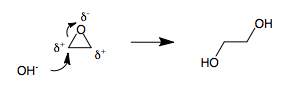
3-membered rings
 3-membered rings are strained moieties in organic chemistry. When a 3-membered ring contains a heteroatom, such as in an
3-membered rings are strained moieties in organic chemistry. When a 3-membered ring contains a heteroatom, such as in an epoxide
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale for ...
or in a bromonium
A halonium ion is any onium ion containing a halogen atom carrying a positive charge. This cation has the general structure where X is any halogen and no restrictions on R, this structure can be cyclic or an open chain molecular structure. Haloni ...
intermediate, the three atoms in the ring become polarized. It is impossible to assign (+) and (-) polarities to a 3-membered ring without having two adjacent atoms with the same polarity. Therefore, whenever a polarized 3-membered ring is opened by a nucleophile, umpolung inevitably results . For example, the opening of ethylene oxide with hydroxide leads to ethylene glycol
Ethylene glycol (IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes, as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odo ...
.
Carbonyl umpolung / anion relay chemistry
Dithiane chemistry is a classic example of polarity inversion. This can be observed in the Corey-Seebach reaction. Ordinarily the oxygen atom in thecarbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
group is more electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
than the carbon atom and therefore the carbonyl group reacts as an electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries ...
at carbon. This polarity can be reversed when the carbonyl group is converted into a dithiane
A dithiane is a heterocyclic compound composed of a cyclohexane core structure wherein two methylene bridges (-- units) are replaced by sulfur centres. The three isomeric parent heterocycles are 1,2-dithiane, 1,3-dithiane and 1,4-dithiane.
1,3 ...
or a thioacetal
In organosulfur chemistry, thioacetals are the sulfur (''thio-'') analogues of acetals (). There are two classes: the less-common monothioacetals, with the formula , and the dithioacetals, with the formula (symmetric dithioacetals) or (asymm ...
. In synthon
In retrosynthetic analysis, a synthon is a hypothetical unit within a target molecule that represents a potential starting reagent in the retroactive synthesis of that target molecule. The term was coined in 1967 by E. J. Corey. He noted in 1 ...
terminology the ordinary carbonyl group is an acyl
In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an alkyl group (). In organic chemistry, the acyl group (IUPAC n ...
cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
and the dithiane is a masked acyl
In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an alkyl group (). In organic chemistry, the acyl group (IUPAC n ...
anion
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
.
When the dithiane is derived from an aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
such as acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the mos ...
the acyl proton can be abstracted by ''n''-butyllithium in THF at low temperatures. The thus generated 2-lithio-1,3-dithiane reacts as a nucleophile in nucleophilic displacement
In chemistry, a nucleophilic substitution is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). The ...
with alkyl halide
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely ...
s such as benzyl bromide
Benzyl bromide is an organic compound with the formula . The molecule consists of a benzene ring substituted with a bromomethyl group. It is a colorless liquid with lachrymatory properties. The compound is a reagent for introducing benzyl groups ...
, with other carbonyl compounds such as cyclohexanone
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has an odor reminiscent of acetone. Over time, samples of cyclohexan ...
or oxirane
Ethylene oxide is an organic compound with the formula . It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly swe ...
s such as phenyl-epoxyethane, shown below. After hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
of the dithiane group the final reaction products are α-alkyl-ketones or α-hydroxy-ketones. A common reagent for dithiane hydrolysis is (bis(trifluoroacetoxy)iodo)benzene.
Dithiane chemistry opens the way to many new chemical transformations. One example is found in so-called anion relay chemistry in which a negative charge of an anionic functional group resulting from one organic reaction is transferred to a different location within the same carbon framework and available for secondary reaction. In this example of a multi-component reaction In chemistry, a multi-component reaction (or MCR), sometimes referred to as a "Multi-component Assembly Process" (or MCAP), is a chemical reaction where three or more compounds
react to form a single product. By definition, multicomponent reaction ...
both formaldehyde
Formaldehyde ( , ) (systematic name methanal) is a naturally occurring organic compound with the formula and structure . The pure compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde (refer to section F ...
(1) and isopropylaldehyde (8) are converted into dithianes 3 and 9 with 1,3-propanedithiol. Sulfide 3 is first silylated by reaction with ''tert''-butyllithium and then trimethylsilyl chloride
Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound ( silyl halide), with the formula (CH3)3SiCl, often abbreviated Me3SiCl or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. ...
4 and then the second acyl proton is removed and reacted with optically active (−)-epichlorohydrin
Epichlorohydrin (abbreviated ECH) is an organochlorine compound and an epoxide. Despite its name, it is not a halohydrin. It is a colorless liquid with a pungent, garlic-like odor, moderately soluble in water, but miscible with most polar organi ...
6 replacing chlorine. This compound serves as the substrate for reaction with the other dithiane 9 to the oxirane
Ethylene oxide is an organic compound with the formula . It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly swe ...
ring opening product 10. Under influence of the polar base HMPA
Hexamethylphosphoramide, often abbreviated HMPA, is a phosphoramide (an amide of phosphoric acid) with the formula This colorless liquid is a useful reagent in organic synthesis.
Structure and reactivity
HMPA is the oxide of the highly basic ...
, 10 rearranges in a 1,4-Brook rearrangement to the silyl ether
Silyl ethers are a group of chemical compounds which contain a silicon atom covalently bonded to an alkoxy group. The general structure is R1R2R3Si−O−R4 where R4 is an alkyl group or an aryl group. Silyl ethers are usually used as protecting g ...
11 reactivating the formaldehyde dithiane group as an anion (hence the anion relay concept). This dithiane group reacts with oxirane 12 to the alcohol 13 and in the final step the sulfide groups are removed with (bis(trifluoroacetoxy)iodo)benzene.
The anion relay chemistry tactic has been applied elegantly in the total synthesis of complex molecules of significant biological activity, such as spongistatin 2 and mandelalide A.
Oxidative bond formation
It is possible to form a bond between two carbons of (-) polarity by using anoxidant
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxi ...
such as iodine
Iodine is a chemical element with the symbol I and atomic number 53. The heaviest of the stable halogens, it exists as a semi-lustrous, non-metallic solid at standard conditions that melts to form a deep violet liquid at , and boils to a vi ...
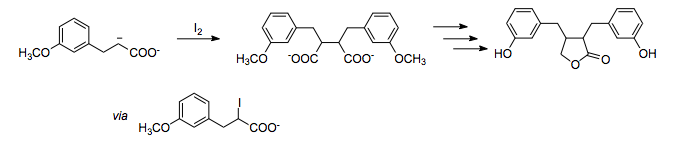
. In this total synthesis of enterolactone
Enterolactone is a organic compound classified as an enterolignan. It is formed by the action of intestinal bacteria on plant lignan precursors present in the diet.
Sources
Many dietary plant lignan precursors, such as secoisolariciresinol, mat ...
, the 1,4- relationship of oxygen substituents is assembled by the oxidative homocoupling of a carboxylate enolate using iodine as the oxidant.

Amine umpolung
Ordinarily the nitrogen atom in theamine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituen ...
group is reacting as a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
by way of its lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
. This polarity can be reversed when a primary or secondary amine is substituted with a good leaving group In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited ...
(such as a halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this group is ...
atom or an alkoxy group
In chemistry, the alkoxy group is an alkyl group which is singularly bonded to oxygen; thus . The range of alkoxy groups is vast, the simplest being methoxy (). An ethoxy group () is found in the organic compound ethyl phenyl ether (, also k ...
). The resulting N-substituted compound can behave as an electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries ...
at the nitrogen atom and react with a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
as for example in the electrophilic amination of carbanion
In organic chemistry, a carbanion is an anion in which carbon is trivalent (forms three bonds) and bears a formal negative charge (in at least one significant resonance form).
Formally, a carbanion is the conjugate base of a carbon acid:
:R3C ...
s.
Hydrazone umpolung
Recently, various carbonyls have been turned into organometallic reagent surrogates via hydrazone umpolung by C.-J. Li et al. In the presence of a catalyst, similar to organometallic reagents, hydrazones can undergo nucleophilic additions, conjugate additions, and transition-metal catalyzed cross-couplings with various electrophiles to form new C-C bonds.References
External links
* {{GoldBookRef , file = U06551 , title = umpolung Organic chemistry