|
Claisen Condensation
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone. It is named after Rainer Ludwig Claisen, who first published his work on the reaction in 1887. Requirements At least one of the reagents must be enolizable (have an α-proton and be able to undergo deprotonation to form the enolate anion). There are a number of different combinations of enolizable and nonenolizable carbonyl compounds that form a few different types of Claisen. The base used must not interfere with the reaction by undergoing nucleophilic substitution or addition with a carbonyl carbon. For this reason, the conjugate sodium alkoxide base of the alcohol formed (e.g. sodium ethoxide if ethanol is formed) is often used, since the alkoxide is regenerated. In mixed Claisen condensations, a non-nucleophilic base such as lithium diisopropylamide, or L ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rainer Ludwig Claisen
Rainer Ludwig Claisen (; 14 January 1851 – 5 January 1930) was a German chemist best known for his work with condensations of carbonyls and sigmatropic rearrangements. He was born in Cologne as the son of a jurist and studied chemistry at the university of Bonn (1869), where he became a member of K.St.V. Arminia. He served in the army as a nurse in 1870–1871 and continued his studies at Georg August University of Göttingen, Göttingen University. He returned to the University of Bonn in 1872 and started his academic career at the same university in 1874. He died in 1930 in Godesberg am Rhein (near Bonn The federal city of Bonn ( lat, Bonna) is a city on the banks of the Rhine in the German state of North Rhine-Westphalia, with a population of over 300,000. About south-southeast of Cologne, Bonn is in the southernmost part of the Rhine-Ru ...). Career Scientific contributions * Described the condensation of aromatic aldehydes with aliphatic aldehydes or ke ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Ethoxide
Sodium ethoxide, also referred to as sodium ethylate, is the ionic, organic compound with the formula , or NaOEt (Et = ethane). It is a white solid, although impure samples appear yellow or brown. It dissolves in polar solvents such as ethanol. It is commonly used as a strong base. Preparation Few procedures have been reported to prepare the anhydrous solid. Instead the material is typically prepared in a solution with ethanol. It is commercially available and as a solution in ethanol. It is easily prepared in the laboratory by treating sodium metal with absolute ethanol: : The reaction of sodium hydroxide with anhydrous ethanol suffers from incomplete conversion to the alkoxide. Structure The crystal structure of sodium ethoxide has been determined by X-ray crystallography. It consists of layers of alternating Na+ and O− centres with disordered ethyl groups covering the top and bottom of each layer. The ethyl layers pack back-to-back resulting in a lamellar structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclic Compound
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon (i.e., are carbocycles), none of the atoms are carbon (inorganic cyclic compounds), or where both carbon and non-carbon atoms are present (heterocyclic compounds). Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size (e.g., < 17 total atoms) numbers in the many b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intramolecular Reaction
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule. Examples * intramolecular hydride transfer (transfer of a hydride ion from one part to another within the same molecule) * intramolecular hydrogen bond (a hydrogen bond formed between two functional groups of the same molecule) *cyclization of ω-haloalkylamines and alcohols to form the corresponding saturated nitrogen and oxygen heterocycles, respectively (an SN2 reaction within the same molecule) In intramolecular organic reactions, two reaction sites are contained within a single molecule. This creates a very high effective concentration (resulting in high reaction rates), and, therefore, many intramolecular reactions that would not occur as an intermolecular reaction between two compounds take place. Examples of intramolecular reactions are the Smiles rearrangement, the Dieckmann condensation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mixed Claisen Example , a person who is of multiple races
*
*
{{disambiguation ...
Mixed is the past tense of ''mix''. Mixed may refer to: * Mixed (United Kingdom ethnicity category), an ethnicity category that has been used by the United Kingdom's Office for National Statistics since the 1991 Census * ''Mixed'' (album), a compilation album of two avant-garde jazz sessions featuring performances by the Cecil Taylor Unit and the Roswell Rudd Sextet See also * Mix (other) * Mixed breed, an animal whose parents are from different breeds or species * Mixed ethnicity Mixed race people are people of more than one race or ethnicity. A variety of terms have been used both historically and presently for mixed race people in a variety of contexts, including ''multiethnic'', ''polyethnic'', occasionally ''bi-ethn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Claisen Ethyl Acetate
Claisen may refer to: *Rainer Ludwig Claisen, a German chemist **Claisen rearrangement, a reaction of a allyl vinyl ether to a γ,δ-unsaturated carbonyl **Claisen condensation, a reaction between esters and carbonyl compounds in the presence of a strong base **Ireland–Claisen rearrangement, a chemical reaction of an allylic ester with strong base **Claisen isatin synthesis Claisen may refer to: *Rainer Ludwig Claisen, a German chemist **Claisen rearrangement, a reaction of a allyl vinyl ether to a γ,δ-unsaturated carbonyl **Claisen condensation, a reaction between esters and carbonyl compounds in the presence of ... See also * 5243 Clasien, a minor planet {{Disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methoxide
In organic chemistry, methoxides are organic salts with a anion. They are the simplest alkoxides. Sodium methoxide and potassium methoxide have widespread use, though other metal-cation variants such as lithium methoxide, rubidium methoxide, and caesium methoxide exist as well. Methoxide ion The methoxide ion has the formula of CH3O− and is the conjugate base of methanol. It is a strong organic base, and since it is stronger than the inorganic hydroxide ion, it can remove a hydrogen atom from a water molecule, yielding methanol and hydroxide. Therefore, methoxide solutions must be kept free of water. Sodium methoxide Sodium methoxide, also called sodium methylate and sodium methanolate, is a white powder when pure. It is used as an initiator of an anionic addition polymerization with ethylene oxide, forming a polyether with high molecular weight. Both sodium methoxide and its counterpart prepared with potassium are frequently used as catalysts for commercial-sc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Group
In organic chemistry, an ethyl group (abbr. Et) is an alkyl substituent with the formula , derived from ethane (). ''Ethyl'' is used in the International Union of Pure and Applied Chemistry's nomenclature of organic chemistry for a saturated two-carbon moiety in a molecule, while the prefix "''eth-''" is used to indicate the presence of two carbon atoms in the molecule. Ethylation Ethylation is the formation of a compound by introduction of the ethyl group. The most widely practiced example of this reaction is the ethylation of benzene with ethylene to yield ethylbenzene, a precursor to styrene, which is a precursor to polystyrene. Approximately 24.7 million tons of ethylbenzene were produced in 1999. :: Many ethyl-containing compounds are generated by electrophilic ethylation, i.e. treatment of nucleophiles with sources of Et+. Triethyloxonium tetrafluoroborate t3OF4 is such a reagent. For good nucleophiles, less electrophilic reagents are employed, such as ethyl h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula . In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bounded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For example, protonation of methanol gives an elect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
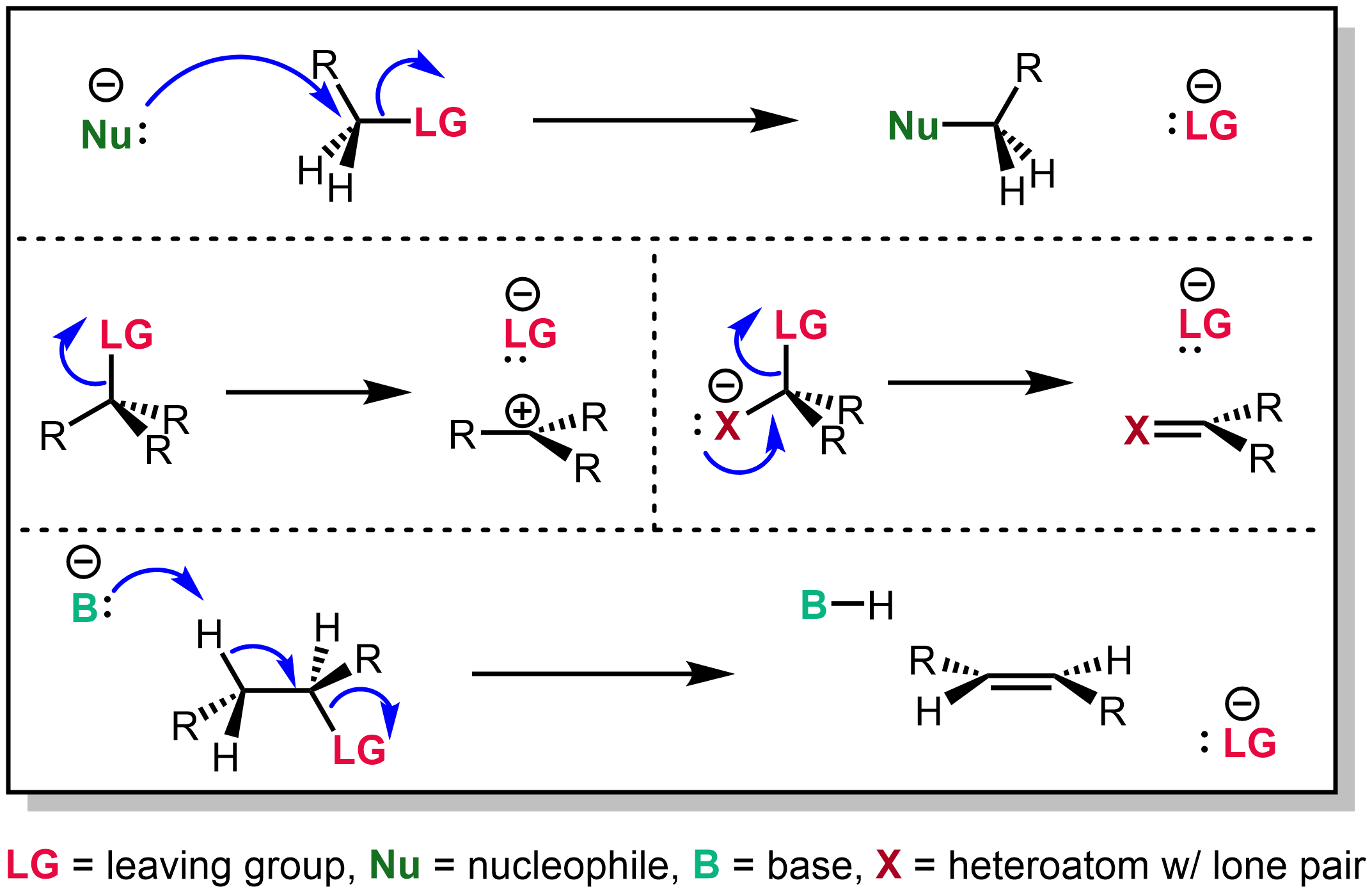
Leaving Group
In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited to a fragment that departs with a pair of electrons in heterolytic bond cleavage. In this usage, a leaving group is a less formal but more commonly used synonym of the term '' nucleofuge''. In this context, leaving groups are generally anions or neutral species, departing from a neutral or cationic substrates, respectively, though in rare cases, cations leaving from a dicationic substrate are also known. A species' ability to serve as a leaving group depends on its ability to stabilize the additional electron density that results from bond heterolysis. Common anionic leaving groups are halides such as Cl−, Br−, and I−, and sulfonate esters such as tosylate (TsO−), while water (H2O), alcohols (HOR), and amines (R3N) are common neutr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries a partial positive charge, or have an atom that does not have an octet of electrons. Electrophiles mainly interact with nucleophiles through addition and substitution reactions. Frequently seen electrophiles in organic syntheses include cations such as H+ and NO+, polarized neutral molecules such as HCl, alkyl halides, acyl halides, and carbonyl compounds, polarizable neutral molecules such as Cl2 and Br2, oxidizing agents such as organic peracids, chemical species that do not satisfy the octet rule such as carbenes and radicals, and some Lewis acids such as BH3 and DIBAL. Organic chemistry Addition of halogens These occur between alkenes and electrophiles, often halogens as in halogen addition reactions. Common reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
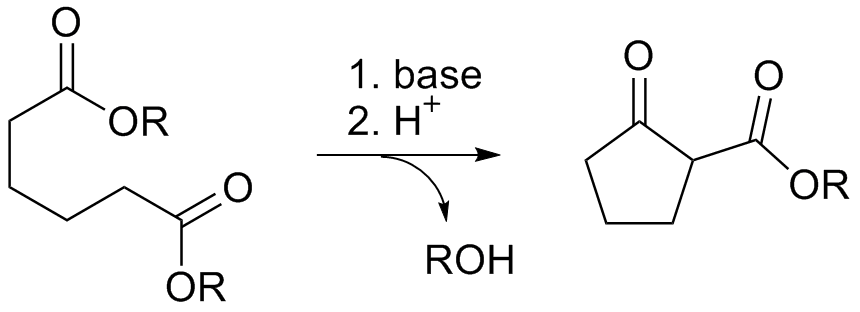
Dieckmann Condensation
The Dieckmann condensation is the intramolecular chemical reaction of diesters with base to give β-keto esters. It is named after the German chemist Walter Dieckmann (1869–1925). The equivalent intermolecular reaction is the Claisen condensation. : Reaction mechanism Deprotonation of an ester at the α-position generates an enolate ion which then undergoes a 5-exo-trig nucleophilic attack to give a cyclic enol. Protonation with a Brønsted-Lowry acid (H3O+ for example) re-forms the β-keto ester. : Due to the steric stability of five- and six-membered rings, these structures will preferentially be formed. 1,6 diesters will form five-membered cyclic β-keto esters, while 1,7 diesters will form six-membered β-keto esters. Further reading *Dieckmann, W. '' Ber.'' 1894, ''27'', 102 & 965 *Dieckmann, W. ''Ber.'' 1900, ''33'', 595 & 2670 *Dieckmann, W. ''Ann.'' 1901, ''317'', 51 & 93 See also * Claisen condensation * Gabriel-Colman rearrangement * Thorpe–Ziegler reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |