triazole on:
[Wikipedia]
[Google]
[Amazon]
A triazole is a
Synthesis of 1,2,3-triazoles (overview of recent methods)
Synthesis of 1,2,4-triazoles (overview of recent methods)
heterocyclic compound
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and ...
featuring a five-membered ring of two carbon atoms and three nitrogen atoms with molecular formula C2H3N3. Triazoles exhibit substantial isomerism
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers.
Iso ...
, depending on the positioning of the nitrogen atoms within the ring.
Many triazoles are versatile, biologically active compounds commonly used as fungicides and plant retardants. However, triazoles are also useful in bioorthogonal chemistry, because the large number of nitrogen atoms causes triazoles to react similar to azides. Lastly, the many free lone pairs in triazoles make them useful as coordination compounds, although not typically as haptic ligands.
Isomerism
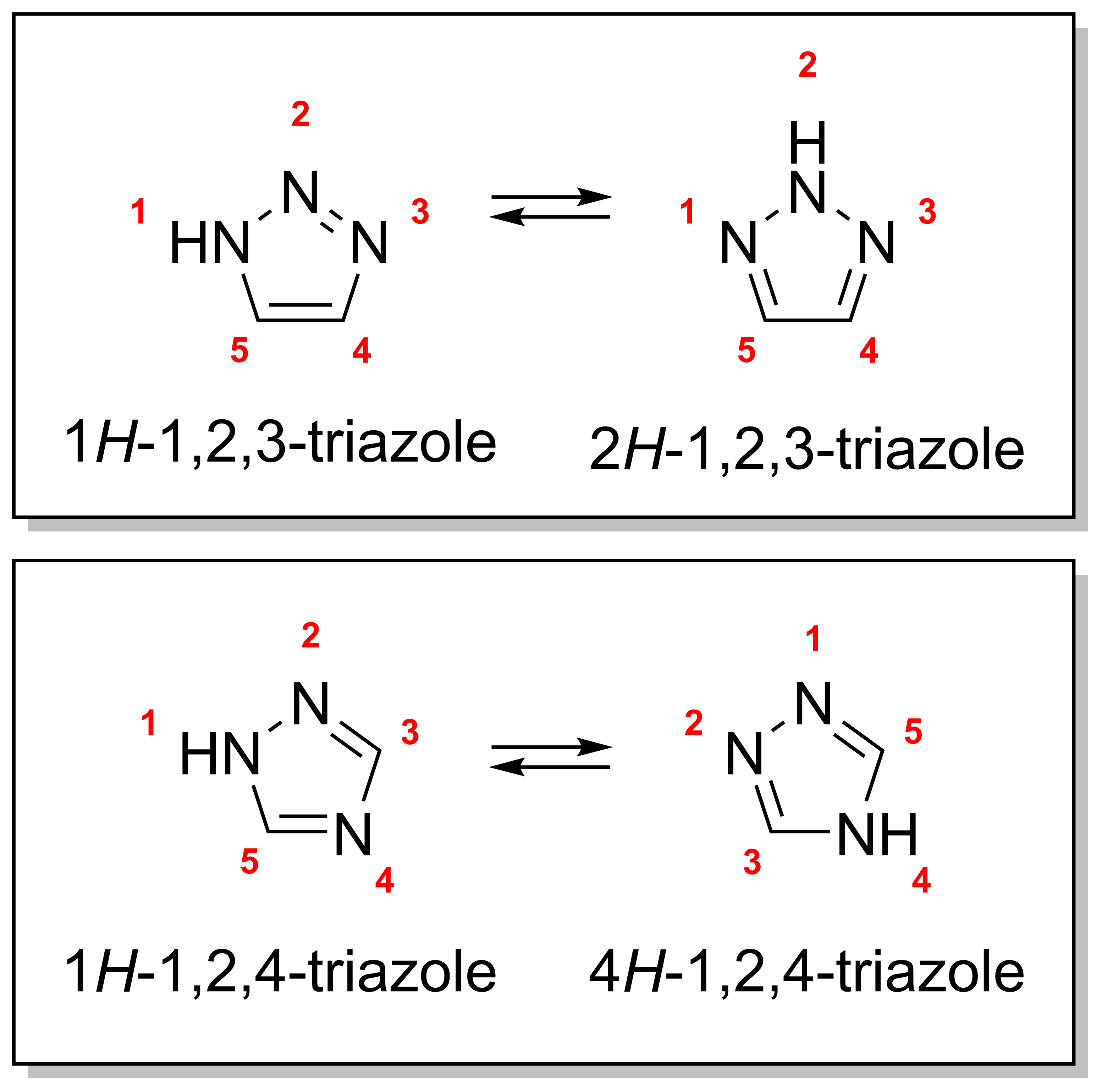
There are four triazole isomers, which are conventionally divided into two pairs of tautomers. In the 1,2,3-triazoles, the three nitrogen atoms are adjacent; in the1,2,4-triazole
1,2,4-Triazole (as ligand in coordination compounds, Htrz abbreviation is sometimes used) is one of a pair of isomeric chemical compounds with molecular formula CHN, called triazoles, which have a five-membered ring of two carbon atoms and three n ...
s, an interstitial carbon separates out one nitrogen atom. Each category has two tautomers that differ by which nitrogen has a hydrogen bonded to it.

Preparation
There are several methods to prepare triazoles.1,2,3-Triazoles
1,2,3-Triazoles are usually prepared following (3+2) cycloaddition protocols. A common technique for unsubstituted triazoles is the Huisgen azide-alkyne 1,3-dipolar cycloaddition: a azide and an alkyne react at high temperature to form a ring. However, the Huisgen strategy produces a mixture of isomers (typically 1,4- and 1,5-disubstituted) when used to produce substituted triazoles. In order to selectively prepare a desired isomer, metal catalysts are employed. In the copper-catalysed azide-alkyne cycloaddition (CuAAC), copper(I) salts select for the formation of 1,4-disubstituted 1,2,3-triazoles. One such catalyst is CuBr(PPh3)3, which is relatively stable towards oxidation even at elevated temperatures and can produce triazoles with a broad range of substituents either in solvent or under ''neat'' reaction conditions. Conversely, ruthenium catalysts (RuAAC) select for 1,5-disubstituted 1,2,3-triazoles.1,2,4-Triazoles
Most techniques for producing 1,2,4-triazoles use the free energy of water, either by dehydrating a mixture of amides and hydrazides (the Pellizzari reaction) or imides and alkyl hydrazines (the Einhorn-Brunner reaction). Of those two, only the Einhorn-Brunner reaction is regioselective. Recent research has focused on grinding and microwave irradiation asgreener Greener is a surname. Notable people with the surname include:
* Bob Greener (1899–1970), English professional footballer
* Christopher Greener (born 1943), United Kingdom's tallest human
* Matthew Greener, British musician
* Richard Theodore Gre ...
substitutes.
Applications
Triazoles are compounds with a vast spectrum of applications, varying from materials (polymers), agricultural chemicals, pharmaceuticals, photoactive chemicals and dyes. Benzotriazole is used in chemical photography as a restrainer and fog suppressant. Cyclohexylethyltriazol was briefly used as an alternative to Cardiazol (Metrazol) in convulsive shock therapy treatment of mental illnesses during the 1940s.Importance in agriculture
Many triazoles have antifungal effects: the triazole antifungal drugs include fluconazole, isavuconazole, itraconazole, voriconazole,pramiconazole
Pramiconazole is a triazole antifungal
An antifungal medication, also known as an antimycotic medication, is a pharmaceutical fungicide or fungistatic used to treat and prevent mycosis such as athlete's foot, ringworm, candidiasis (thrush), s ...
, ravuconazole
Ravuconazole (codenamed BMS-207147 and ER-30346) is a potent triazole antifungal, the development of which was discontinued in 2007. The drug has shown to have a similar spectrum of activity to voriconazole, with an increased half-life. Howev ...
, and posaconazole and triazole plant-protection fungicides include epoxiconazole, , myclobutanil, propiconazole, prothioconazole
Prothioconazole is a synthetic chemical produced primarily for its fungicidal properties. It is a member of the class of compounds triazoles, and possesses a unique toxophore in this class of fungicides. Its effective fungicidal properties can be ...
, metconazole, cyproconazole, tebuconazole, flusilazole and paclobutrazol.
Due to spreading resistance of plant pathogens towards fungicides of the strobilurin class, control of fungi such as ''Septoria tritici
''Zymoseptoria tritici'', synonyms ''Septoria tritici'', ''Mycosphaerella graminicola'', is a species of filamentous fungus, an ascomycete in the family ''Mycosphaerellaceae''. It is a wheat plant pathogen causing septoria leaf blotch that is ...
'' or ''Gibberella zeae
''Gibberella zeae'', also known by the name of its anamorph ''Fusarium graminearum'', is a fungal plant pathogen which causes fusarium head blight (FHB), a devastating disease on wheat and barley. The pathogen is responsible for billions of dolla ...
'' relies heavily on triazoles. Food, like store bought potatoes, contain retardants such as triazole or tetcyclacis.
In addition, paclobutrazol, uniconazole
Uniconazole is a triazole chemical used as a plant growth retardant. It is active on a wide range of plants and acts by inhibiting the production of gibberellins.
Uses
Uniconazole is applied to plants to restrain their growth. It is often used ...
, , and triadimefon
Triadimefon is a fungicide used in agriculture to control various fungal diseases. As a seed treatment, it is used on barley, corn, cotton, oats, rye, sorghum, and wheat. In fruit it is used on pineapple and banana. Non-food uses include pine ...
are used as plant growth retardants. Brassinazole inhibits brassinosteroid biosynthesis.
Importance in chemical synthesis
Theazide alkyne Huisgen cycloaddition
The azide-alkyne Huisgen cycloaddition is a 1,3-dipolar cycloaddition between an azide and a terminal or internal alkyne to give a 1,2,3-triazole. Rolf Huisgen was the first to understand the scope of this organic reaction. American chemist K ...
is a mild and selective reaction that gives 1,2,3-triazoles as products. The reaction has been widely used in bioorthogonal chemistry and in organic synthesis. Triazoles are relatively stable functional groups and triazole linkages can be used in a variety of applications, e.g. replacing the phosphate backbone of DNA.
Related heterocycles
* Imidazole, an analog with two nonadjacent nitrogen atoms * Pyrazole, an analog with two adjacent nitrogen atoms * Tetrazole, an analog with four nitrogen atoms *Triazolium salt
Triazolium salts are chemical compounds based on the Substitution reaction, substituted triazole structural element. They are composed of a cation based on a heterocyclic compound, heterocyclic five-membered ring with three nitrogen atoms, two o ...
s, substituted analogues that can be used as NHC NHC could refer to:
* Nag Hammadi Codex, or Nag Hammadi Codices (e.g. NHC II, NHC XIII)
* New Hanover County, a county in North Carolina
* New Haven County, a county in Connecticut.
* The National Humanities Center in North Carolina
* The Nationa ...
precursors
External links
Synthesis of 1,2,3-triazoles (overview of recent methods)
Synthesis of 1,2,4-triazoles (overview of recent methods)
References
{{Authority control Chemical compounds Simple aromatic rings Triazoles