Chemical development
Because these alloys are intended for high temperature applications (i.e. holding their shape at temperatures near their melting point) theirActive research
While Ni-based superalloys are excellent high temperature materials and have proven very useful, Co-based superalloys potentially possess superior hot corrosion, oxidation, and wear resistance as compared to Ni-based superalloys. For this reason, efforts have also been put into developing Co-based superalloys over the past several years. Despite that, traditional Co-based superalloys have not found widespread usage because they have a lower strength at high temperature than Ni-based superalloys. The main reason for this is that—until recently—they appeared to lack the γ’ precipitation strengthening that is so important in the high temperature strength of Ni-based superalloys. A 2006 report on metastable γ’-Co3(Al,W) intermetallic compound with the L12 structure suggests Co-based alloys as alternative to traditional Ni-based superalloys. However this class of alloys was reported in a PhD thesis by C. S. Lee in 1971. The two-phase microstructure consists of cuboidal γ’ precipitates embedded in a continuous γ matrix and is therefore morphologically identical to the microstructure observed in Ni-based superalloys. Like in the Ni-based system, there is a high degree of coherency between the two phases, which is one of the main factors resulting in the superior strength at high temperatures. This provides a pathway for the development of a new class of load-bearing Co-based superalloys for application in severe environments. In these alloys, W is the crucial addition for forming the γ’ intermetallic compound; this makes them much denser (>9.6 g/cm3) compared to Ni-based superalloys. Recently a new class of γ - γ’ cobalt-based superalloys have been developed that are W-free and have much lower density comparable to nickel-based superalloys. In addition to the fact that many of the properties of these new Co-based superalloys could be better than those of the more traditional Ni-based ones, Co also has a higher melting temperature than Ni. Therefore, if the high temperature strength could be improved, the development of novel Co-based superalloys could allow for an increase in jet engine operation temperature resulting in an increased efficiency.Phase formation
Adding new elements is usually good because of solid solution strengthening, but engineers need to be careful about which phases precipitate. Precipitates can be classified as geometrically close-packed (GCP), topologically close-packed (TCP), or carbides. GCP phases are usually good for mechanical properties, but TCP phases are often deleterious. Because TCP phases are not truly close packed, they have few slip systems and are very brittle. They are additionally bad because they "scavenge" elements away from GCP phases. Many elements that are good for forming γ' or have great solid solution strengthening may precipitate TCPs. Engineers need to find the balance that promotes GCPs while avoiding TCPs. An area of the alloy with TCP phase formation will be weak because: * the TCP phase has inherently poor mechanical properties * the TCP phase is incoherent with the γ matrix * the TCP phase is surrounded by a "depletion zone" where there is no γ' * the TCP phase usually forms sharp plate or needle-like morphologies which easily nucleate cracks The main GCP phase is γ'. Almost all superalloys are Ni-based because of this phase. γ' is an ordered L1 (pronounced L-one-two), which means it has a certain atom on the face of the unit cell, and a certain atom on the corners of the unit cell. For Ni-based superalloys, that usually means Ni on the faces and Ti or Al on the corners. Another "good" GCP phase is γFamilies of superalloys
History and development of Ni-based superalloys
The United States became interested in gas turbine development around 1905. From 1910-1915, austenitic ( γ phase) stainless steels were developed for the high temperatures in gas turbines. By 1929, 80Ni-20Cr alloy was the norm, with small additions of Ti and Al. Although early metallurgists did not know it yet, they were forming small γ' precipitates in Ni-based superalloys. These alloys quickly surpassed Fe- and Co-based superalloys, which were strengthened by carbides and solid solution strengthening. Although Cr was great for protecting the alloys from oxidation and corrosion up to 700 °C, metallurgists began decreasing Cr in favor of Al, which had oxidation resistance at much higher temperatures. The lack of Cr caused issues with hot corrosion, so coatings needed to be developed. Around 1950, vacuum melting became commercialized, which allowed metallurgists to create higher purity alloys with more precise composition. In the 60s and 70s, metallurgists changed focus from alloy chemistry to alloy processing. Directional solidification was developed to allow columnar or even single-crystal turbine blades. Oxide dispersion strengthening could obtain very fine grains and superplasticity.Ni-based superalloy phases
* Gamma (γ): This phase composes the matrix of Ni-based superalloy. It is a solid solution fcc austenitic phase of the alloying elements. Alloying elements found in most commercial Ni-based alloys are, C, Cr, Mo, W, Nb, Fe, Ti, Al, V, and Ta. During the formation of these materials, as the Ni-alloys are cooled from the melt, carbides begin to precipitate, at even lower temperatures γ' phase precipitates. * Gamma prime (γ'): This phase constitutes the precipitate used to strengthen the alloy. It is an intermetallic phase based on Ni3(Ti,Al) which have an ordered FCC L12 structure. The γ' phase is coherent with the matrix of the superalloy having a lattice parameter that varies by around 0.5%. Ni3(Ti,Al) are ordered systems with Ni atoms at the cube faces and either Al or Ti atoms at the cube edges. As particles of γ' precipitates aggregate, they decrease their energy states by aligning along the <100> directions forming cuboidal structures. This phase has a window of instability between 600 °C and 850 °C, inside of which γ' will transform into the HCP η phase. For applications at temperatures below 650 °C, the γ" phase can be utilized for strengthening.History and development of Co-based superalloys
Historically, Co-based superalloys have depended on carbide precipitation and solid solution strengthening for mechanical properties. While these strengthening mechanisms are inferior to gamma prime (γ') precipitation strengthening, cobalt has a higher melting point than currently ubiquitous nickel-based superalloys and has superior hot corrosion resistance and thermal fatigue. As a result, carbide-strengthened Co-based superalloys are used in lower stress, higher temperature applications such as stationary vanes in gas turbines. However, recent research has shown that cobalt ''can'' exhibit the γ' phase. Actually, the first reported existence of γ' occurred in a 1971 PhD dissertation, but was never published. The γ/γ' microstructure was rediscovered and first published in 2006 by Sato et al. That γ' phase was Co3(Al, W). It was furthermore found that Mo, Ti, Nb, V, and Ta partition to the γ' phase, while Fe, Mn, and Cr partition to the matrix γ. The next family of Co-based superalloys was discovered in 2015 by Makineni et al. This family has a similar γ/γ' microstructure, but is tungsten-free and has a γ' phase of Co3(Al,Mo,Nb). Since tungsten is a very heavy element, the elimination of tungsten makes Co-based alloys increasingly viable in turbines for aircraft, where low density is especially important. The most recently discovered family of superalloys was computationally predicted in a high throughput study by Nyshadham et al. in 2017, and demonstrated in the lab by Reyes Tirado et al. in 2018. This γ' phase is again tungsten free and has the composition Co3(Nb,V) and Co3(Ta,V).Co-based superalloy phases
*Gamma (γ): As in Ni-based superalloys, this is the matrix phase. While Co-based superalloys are not used commercially to the extent of Ni-based superalloys, alloying elements found in research Co-based alloys are C, Cr, W, Ni, Ti, Al, Ir, and Ta. As in stainless steels, Chromium is used (occasionally up to 20 wt.%) to improve resistance to oxidation and corrosion via the formation of a Cr2O3 passive layer, which is critical for use in gas turbines, but also provides solid-solution strengthening due to the mismatch in the atomic radii of Co and Cr, and precipitation hardening due to the formation of MC-type carbides. * Gamma Prime (γ'): As in Ni-based superalloys, this phase constitutes the precipitate used to strengthen the alloy. It is usually close-packed with a L12 structure of Co3Ti or FCC Co3Ta, though both W and Al have been found to integrate into these cuboidal precipitates quite well. The elements Ta, Nb, and Ti integrate into the γ’ phase and are quite effective at stabilizing it at high temperatures. * Carbide Phases: As is common with carbide formation, carbides strengthen the alloy through precipitation hardening but decrease low-temperature ductility. * Topologically Close-Packed (TCP) phases may also appear in some developmental Co-based superalloys, but embrittle the alloy and are thus undesirable.Fe-based superalloy phases
The use of steels in superalloy applications is of interest because certain steel alloys have showed creep and oxidation resistance similar to that of Ni-based superalloys, while being far less expensive to produce. Gamma (γ): Like the phases found in Ni-based superalloys, Fe-based alloys feature a matrix phase of austenite iron (FCC). Alloying elements that are commonly found in these stainless steel alloys include: Al, B, C, Co, Cr, Mo, Ni, Nb, Si, Ti, W, and Y. While Al is introduced for its oxidation benefits, Al additions must be kept at low weight fractions (wt.%) because Al stabilizes a ferritic (BCC) primary phase matrix, which is an undesirable phase in superalloy microstructures, as it is inferior to the high temperature strength exhibited by an austenitic (FCC) primary phase matrix. Gamma-prime (γ’): This phase is introduced as precipitates to strengthen the alloy. Like in Ni-based alloys, γ’-Ni3Al precipitates can be introduced with the proper balance of Al, Ni, Nb, and Ti additions.Microstructure of Fe-based superalloys
Two major types of austenitic stainless steels exist and are characterized by the oxide layer that forms at the surface of the steel: chromia-forming or alumina-forming stainless steel. Chromia-forming stainless steel is the most common type of stainless steel produced. However, chromia-forming steels do not exhibit high creep resistance at high operating temperatures, especially in environments with water vapor, when compared to Ni-based superalloys. Exposure to water vapor at high operating temperatures can result in an increase in internal oxidation in chromia-forming alloys and rapid formation of volatile Cr (oxy)hydroxides, both of which can reduce the durability and lifetime of the alloy. Alumina-forming austenitic stainless steels feature a single-phase matrix of austenite iron (FCC) with an alumina oxide at the surface of the steel. Alumina is more thermodynamically stable in oxygen than chromia. More commonly, however, precipitate phases are introduced to increase strength and creep resistance. In alumina-forming steels, NiAl precipitates are introduced to act as Al reservoirs to maintain the protective alumina layer. In addition, Nb and Cr additions help form and stabilize alumina by increasing precipitate volume fractions of NiAl. Research endeavors for the development of alumina-forming, Fe-base superalloys have shown at least 5 grades of alumina-forming austenitic (AFA) alloys, with different operating temperatures at oxidation in air + 10% water vapor: * AFA Grade: (50-60)Fe-(20-25)Ni-(14-15)Cr-(2.5-3.5)Al-(1-3)Nb wt.% base ** 750-800 °C operating temperatures at oxidation in air + 10% water vapor * Low Nickel AFA Grade: 63Fe-12Ni-14Cr-2.5Al-0.6Nb-5Mn3Cu wt.% base ** 650 °C operating temperatures at oxidation in air + 10% water vapor * High Performance AFA Grade: (45-55)Fe-(25-30)Ni-(14-15)Cr(3.5-4.5)Al-(1-3)Nb-(0.02-0.1)Hf/Y wt.% base ** 850-900 °C operating temperatures at oxidation in air + 10% water vapor * Cast AFA Grade: (35-50)Fe-(25-35)Ni-14Cr-(3.5-4)Al-1Nb wt.% base ** 750-1100 °C operating temperatures at oxidation in air + 10% water vapor, depending upon Ni wt.% * AFA superalloy (40-50)Fe-(30-35)Ni-(14-19)Cr-(2.5-3.5)Al-3Nb ** 750-850 °C operating temperatures at oxidation in air + 10% water vapor Operating temperatures with oxidation in air and no water vapor are expected to be higher. In addition, an AFA superalloy grade was shown to exhibit a creep strength approaching that of the nickel-based alloy UNS N06617.Microstructure of superalloys
In pure Ni3Al phaseSingle-crystal superalloys
Single-crystal superalloys (SX or SC superalloys) are formed as a single crystal using a modified version of the directional solidification technique, so there are no grain boundaries in the material. The mechanical properties of most other alloys depend on the presence of grain boundaries, but at high temperatures, they would participate inOxidation in superalloys
For superalloys operating at high temperatures and exposed to corrosive environments, the oxidation behavior is of paramount concern. Oxidation involves chemical reactions of the alloying elements with oxygen to form newSuperalloy processing
The historical developments in superalloy processing have brought about considerable increases in superalloyCasting and forging
Casting and forging are traditional metallurgical processing techniques that can be used to generate both polycrystalline and monocrystalline products. Polycrystalline casts tend to have higher fracture resistance, while monocrystalline casts have higher creep resistance. Jet turbine engines employ both poly and mono crystalline components to take advantage of their individual strengths. The disks of the high-pressure turbine, which are near the central hub of the engine are polycrystalline. The turbine blades, which extend radially into the engine housing, experience a much greater centripetal force, necessitating creep resistance. As a result, turbine blades are typically monocrystalline or polycrystalline with a preferred crystal orientation.Investment casting
Investment casting is a metallurgical processing technique in which a wax form is fabricated and used as a template for a ceramic mold. Briefly, a ceramic mold is poured around the wax form, the wax form is melted out of the ceramic mold, and molten metal is poured into the void left by the wax. This leads to a metal form in the same shape as the original wax form. Investment casting leads to a polycrystalline final product, as nucleation and growth of crystal grains occurs at numerous locations throughout the solid matrix. Generally, the polycrystalline product has no preferred grain orientation.Directional solidification
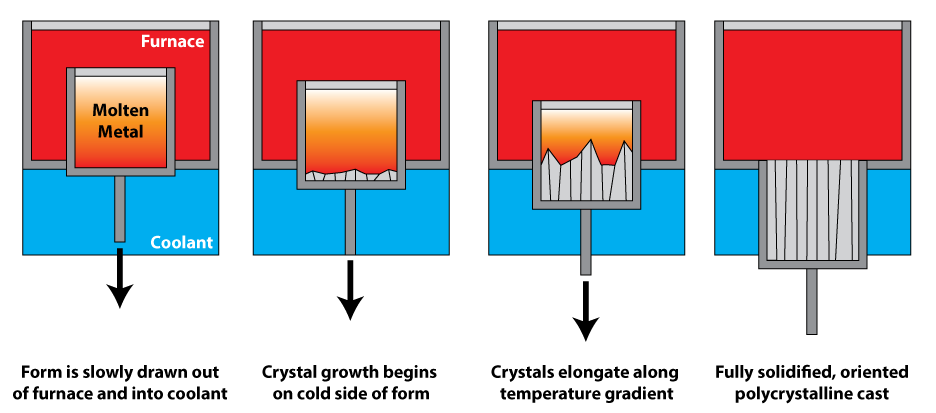 Directional solidification uses a thermal gradient to promote nucleation of metal grains on a low temperature surface, as well as to promote their growth along the temperature gradient. This leads to grains elongated along the temperature gradient, and significantly greater creep resistance parallel to the long grain direction. In polycrystalline turbine blades, directional solidification is used to orient the grains parallel to the centripetal force. It is also known as dendritic solidification.
Directional solidification uses a thermal gradient to promote nucleation of metal grains on a low temperature surface, as well as to promote their growth along the temperature gradient. This leads to grains elongated along the temperature gradient, and significantly greater creep resistance parallel to the long grain direction. In polycrystalline turbine blades, directional solidification is used to orient the grains parallel to the centripetal force. It is also known as dendritic solidification.
Single crystal growth
Single crystal growth starts with a seed crystal which is used to template growth of a larger crystal. The overall process is lengthy, and additional processing via machining is necessary after the single crystal is grown.Powder metallurgy
Powder metallurgy is a class of modern processing techniques in which metals are first converted into a powdered form, and then formed into the desired shape by heating below the melting point. This is in contrast to casting, which occurs with molten metal. Superalloy manufacturing often employs powder metallurgy because of its material efficiency - typically much less waste metal must be machined away from the final product—and its ability to facilitate mechanical alloying. Mechanical alloying is a process by which reinforcing particles are incorporated into the superalloy matrix material by repeated fracture and welding.Sintering and hot isostatic pressing
Sintering and hot isostatic pressing are processing techniques used to densify materials from a loosely packed " green body" into a solid object with physically merged grains. Sintering occurs below the melting point, and causes adjacent particles to merge at their boundaries, leading to a strong bond between them. In hot isostatic pressing, a sintered material is placed in a pressure vessel and compressed from all directions (isostatically) in an inert atmosphere to affect densification.Additive manufacturing
Selective laser melting (also known as ''powder bed fusion'') is an additive manufacturing procedure used to create intricately detailed forms from a CAD file. In CAD, a shape is designed and then converted into slices. These slices are sent to a laser writer to print the final product. In brief, a bed of metal powder is prepared, and the first slice of the CAD design is formed in the powder bed by a high energy laser sintering the particles together. After this first slice is generated, the powder bed moves downwards, and a new batch of metal powder is rolled over the top of the slice. The second layer is then sintered with the laser, and the process is repeated until all the slices in the CAD file have been processed. Due to the nature of many additive manufacturing processes, porosity can be present in products made by selective laser melting. Many products will often undergo a heat treatment or hot isostatic pressing procedure to densify the product and reduce porosity which can result in cracking. Additive manufacturing for these applications is therefore particularly challenging.Coating of superalloys
In modern gas turbine, the turbine entry temperature (~1750K) has exceeded the incipient melting temperature of superalloys (~1600K), with the help of surface engineering. Under such extreme working condition, the qualification of coating becomes vital.Different types of coating
Historically, three "generations" of coatings have been developed: diffusion coatings, overlay coatings and thermal barrier coatings. Diffusion coatings, mainly constituted with aluminide or platinum-aluminide, is still the most common form of surface protection. To further enhance resistance to corrosion and oxidation, MCrAlX-based overlay coatings (M=Ni or Co, X=Y, Hf, Si) are deposited to surface of superalloys. Compared to diffusion coatings, overlay coatings are less dependent on the composition of the substrate, but also more expensive, since they must be carried out by air or vacuum plasma spraying (APS/VPS) or else electron beam physical vapour deposition (EB-PVD). Thermal barrier coatings provide by far the best enhancement in working temperature and coating life. It is estimated that modern TBC of thickness 300 μm, if used in conjunction with a hollow component and cooling air, has the potential to lower metal surface temperatures by a few hundred degrees.Thermal barrier coatings
Thermal barrier coatings (TBCs) are used extensively on the surface of superalloy in both commercial and military gas turbine engines to increase component life and engine performance. A coating of about 1-200 µm can reduce the temperature at the superalloy surface by up to 200K. TBCs are really a system of coatings consisting of a bond coat, a thermally grown oxide (TGO), and a thermally insulating ceramic top coat. In most applications, the bond coat is either a MCrAlY (where M=Ni or NiCo) or a Pt modified aluminide coating. A dense bond coat is required to provide protection of the superalloy substrate from oxidation and hot corrosion attack and to form an adherent, slow growing TGO on its surface. The TGO is formed by oxidation of the aluminum that is contained in the bond coat. The current (first generation) thermal insulation layer is composed of 7wt % yttria-stabilized zirconia (7YSZ) with a typical thickness of 100–300 µm. Yttria stabilized zirconia is used due to its low thermal conductivity (2.6W/mK for fully dense material), relatively high coefficient of thermal expansion, and good high temperature stability. The electron beam directed vapor deposition (EB-DVD) process used to apply the TBC to turbine airfoils produces a columnar microstructure with several levels of porosity. The porosity between the columns is critical to providing strain tolerance (via a very low in-plane modulus), as it would otherwise spall on thermal cycling due to thermal expansion mismatch with the superalloy substrate. The porosity within the columns reduces the thermal conductivity of the coating.Bond coat
The bond coat adheres the thermal barrier coating to the superalloy substrate. Additionally, the bond coat provides oxidation protection and functions as a diffusion barrier against the motion of substrate atoms towards the environment. There are five major types of bond coats, the aluminides, the platinum-aluminides, MCrAlY, cobalt-cermets, and nickel-chromium. For the aluminide bond coatings, the final composition and structure of the coating depends on the composition of the substrate. Aluminides also lack ductility below 750 °C, and exhibit a limited by thermomechanical fatigue strength. The Pt-aluminides are very similar to the aluminide bond coats except for a layer of Pt (5—10 μm) deposited on the blade. The Pt is believed to aid in oxide adhesion and contributes to hot corrosion. The cost of Pt plating is justified by the increased blade life span. The MCrAlY is the latest generation of bond coat and does not strongly interact with the substrate. Normally applied by plasma spraying, MCrAlY coatings are secondary aluminum oxide formers. This means that the coatings form an outer layer of chromium oxide (chromia), and a secondary aluminum oxide (alumina) layer underneath. These oxide formations occur at high temperatures in the range of those that superalloys usually encounter. The chromia provides oxidation and hot-corrosion resistance. The alumina controls oxidation mechanisms by limiting oxide growth by self-passivating. The yttrium enhances the oxide adherence to the substrate, and limits the growth of grain boundaries (which can lead to flaking of the coating). Investigation indicates that addition of rhenium and tantalum increases oxidation resistance. Cobalt-cermet-based coatings consisting of materials such as tungsten carbide/cobalt can be used due to excellent resistance to abrasion, corrosion, erosion, and heat. These cermet coatings perform well in situations where temperature and oxidation damage are significant concerns, such as boilers. One of the unique advantages of cobalt cermet coatings is a minimal loss of coating mass over time, due to the strength of carbides within the mixture. Overall, cermet coatings are useful in situations where mechanical demands are equal to chemical demands for superalloys. Nickel-chromium coatings are used most frequently in boilers fed byProcess methods of coating
Superalloy products that are subjected to high working temperatures and corrosive atmosphere (such as high-pressure turbine region of jet engines) are coated with various kinds of coating. Several kinds of coating process are applied: pack cementation process, gas phase coating (both are a type ofPack cementation process
Pack cementation is a widely used chemical vapor deposition technique which consists of immersing the components to be coated in a metal powder mixture and ammonium halide activators and sealing them in a retort. The entire apparatus is placed inside a furnace and heated in a protective atmosphere to a lower than normal temperature for diffusion to take place, due to the halide salts chemical reaction which causes a eutectic bond between the two metals. The new surface alloy that is formed due to thermal diffused ion migration has a metallurgical bond to the surface substrate and a true intermetallic layer found in the gamma layer of the new surface alloys. The traditional pack consists of four components: Substrate or parts- Ferrous and non-ferrous Powdered alloy- (Ti and/or Al, Si and/or Zn, B and/ or Cr) Halide salt activator- Ammonium halide salts Relatively inert filler powder (Al2O3, SiO2, or SiC) Temperatures below (750 °C) This process includes but is not limited to: Aluminizing Chromizing Siliconizing Sherardizing Boronizing Titaniumizing Pack Cementation has had a revival in the last 10 years as it is being combined with other chemical processes to lower the temperatures of metal combinations even further and give intermetallic properties to different alloy combinations for surface treatments.Thermal spraying
Thermal spraying is a process of applying coatings by heating a feedstock of precursor material and spraying it on a surface. Different specific techniques are used depending on desired particle size, coat thickness, spray speed, desired area, etc. The coatings applied by thermal spraying of any kind, however, rely on adhesion to the surface. As a result, the surface of the superalloy must be cleaned and prepared, usually polished, before application of the thermal coating.Plasma spraying
Of the various thermal spray methods, one of the more ideal and commonly used techniques for coating superalloys isGas phase coating
This process is carried out at higher temperatures, about 1080 °C. The coating material is usually loaded onto special trays without physical contact with the parts to be coated. The coating mixture contains active coating material and activator, but usually does not contain thermal ballast. As in the pack cementation process, the gaseous aluminium chloride (or fluoride) is transferred to the surface of the part. However, in this case the diffusion is outwards. This kind of coating also requires diffusion heat treatment.Failure mechanisms in thermal barrier coating systems
Failure of thermal barrier coating usually manifests as delamination, which arises from the temperature gradient during thermal cycling between ambient temperature and working conditions coupled with the difference in thermal expansion coefficient of the substrate and coating. It is rare for the coating to fail completely – some pieces of it remain intact, and significant scatter is observed in the time to failure if testing is repeated under identical conditions. There are various degradation mechanisms for thermal barrier coating, and some or all of these must operate before failure finally occurs: * Oxidation at the interface of thermal barrier coating and underlying bond coat; * The depletion of aluminum in bond coat due to oxidation and diffusion with substrate; * Thermal stresses from mismatch in thermal expansion coefficient and growth stress due to the formation of thermally grown oxide layer; * Imperfections near thermally grown oxide layer; * Various other complicating factors during engine operation. Additionally, TBC life is very dependent upon the combination of materials (substrate, bond coat, ceramic) and processes (EB-PVD, plasma spraying) used.Applications
Turbines
Nickel-based superalloys are used in load-bearing structures to the highest homologous temperature of any common alloy system (Tm = 0.9, or 90% of their melting point). Among the most demanding applications for a structural material are those in the hot sections of turbine engines (e.g. turbine blade). The preeminence of superalloys is reflected in the fact that they currently comprise over 50% of the weight of advanced aircraft engines. The widespread use of superalloys in turbine engines coupled with the fact that the thermodynamic efficiency of turbine engines is increased with increasing turbine inlet temperatures has, in part, provided the motivation for increasing the maximum-use temperature of superalloys. In fact, during the past 30 years turbine airfoil temperature capability has increased on average by about 4 °F (2.2 °C) per year. Two major factors which have made this increase possible are: # Advanced processing techniques, which improved alloy cleanliness (thus improving reliability) and/or enabled the production of tailored microstructures such as directionally solidified or single-crystal material. # Alloy development resulting in higher-use-temperature materials primarily through the additions of refractory elements such as Re, W, Ta, and Mo. About 60% of the use-temperature increases have occurred due to advanced cooling concepts; 40% have resulted from material improvements. State-of-the-art turbine blade surface temperatures are near ; the most severe combinations of stress and temperature corresponds to an average bulk metal temperature approaching . Although Nickel-based superalloys retain significant strength to temperatures near , they tend to be susceptible to environmental attack because of the presence of reactive alloying elements (which provide their high-temperature strength). Surface attack includes oxidation, hot corrosion, and thermal fatigue. In the most demanding applications, such as turbine blade and vanes, superalloys are often coated to improve environmental resistance. In general, high temperature materials are needed for energy conversion and energy production applications. MaximumResearch and development of new superalloys
The availability of superalloys during past decades has led to a steady increase in turbine entry temperatures, and the trend is expected to continue. Sandia National Laboratories is studying a new method for making superalloys, known asSee also
* Oxide dispersion strengthened alloy * Titanium aluminideReferences
Bibliography
* *External links
* {{cite web , url=http://www.msm.cam.ac.uk/phase-trans/2003/nickel.html , title=Superalloys , publisher=Cambridge University Extensive bibliography and links. Metallurgy Aerospace materials Emerging technologies