Stabilizers For Polymers on:
[Wikipedia]
[Google]
[Amazon]
Polymer stabilizers (British: polymer stabilisers) are chemical additives which may be added to
 Primary antioxidants (also known as chain-breaking antioxidants) act as
Primary antioxidants (also known as chain-breaking antioxidants) act as
 Secondary antioxidants act to remove
Secondary antioxidants act to remove
 Antiozonants prevent or slow down the degradation of material caused by
Antiozonants prevent or slow down the degradation of material caused by
 Light stabilizer are used to inhibit polymer photo-oxidation, which is the combined result of the action of light and oxygen. Like
Light stabilizer are used to inhibit polymer photo-oxidation, which is the combined result of the action of light and oxygen. Like
 Photo-oxidation can begin with the absorption of light by a
Photo-oxidation can begin with the absorption of light by a
 The ability of hindered amine light stabilizers (HALS or HAS) to scavenge radicals produced by weathering, may be explained by the formation of aminoxyl radicals through a process known as the Denisov Cycle. The aminoxyl radical (N-O•) combines with free radicals in polymers:
N-O• + R• → N-O-R
Although they are traditionally considered as light stabilizers, they can also stabilize thermal degradation.
Even though HALS are extremely effective in polyolefins,
The ability of hindered amine light stabilizers (HALS or HAS) to scavenge radicals produced by weathering, may be explained by the formation of aminoxyl radicals through a process known as the Denisov Cycle. The aminoxyl radical (N-O•) combines with free radicals in polymers:
N-O• + R• → N-O-R
Although they are traditionally considered as light stabilizers, they can also stabilize thermal degradation.
Even though HALS are extremely effective in polyolefins,
polymer
A polymer (; Greek ''poly-'', "many" + '' -mer'', "part")
is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and ...
ic materials, such as plastic
Plastics are a wide range of synthetic or semi-synthetic materials that use polymers as a main ingredient. Their plasticity makes it possible for plastics to be moulded, extruded or pressed into solid objects of various shapes. This adaptab ...
s and rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Thailand, Malaysia, a ...
s, to inhibit or retard their degradation.
Common polymer degradation
Polymer degradation is the reduction in the physical properties of a polymer, such as strength, caused by changes in its chemical composition. Polymers and particularly plastics are subject to degradation at all stages of their product life cycl ...
processes include oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a ...
, UV-damage, thermal degradation
Thermal decomposition, or thermolysis, is a chemical decomposition
Chemical decomposition, or chemical breakdown, is the process or effect of simplifying a single chemical entity (normal molecule, reaction intermediate, etc.) into two or more ...
, ozonolysis
In organic chemistry, ozonolysis is an organic reaction where the unsaturated bonds of alkenes (), alkynes (), or azo compounds () are cleaved with ozone (). Alkenes and alkynes form organic compounds in which the multiple carbon–carbon bon ...
, combinations thereof such as photo-oxidation, as well as reactions with catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
residues, dyes, or impurities.
All of these degrade the polymer at a chemical level, via chain scission, uncontrolled recombination and cross-link
In chemistry and biology a cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds and the polymers can be either synthetic polymers or natural ...
ing, which adversely affects many key properties such as strength, malleability
Ductility is a mechanical property commonly described as a material's amenability to drawing (e.g. into wire). In materials science, ductility is defined by the degree to which a material can sustain plastic deformation under tensile stres ...
, appearance and colour.
Stabilizers are used at all stages of the polymer life-cycle. They allow plastic items to be produced faster and with fewer defects, extend their useful lifespan, and facilitate their recycling. However they also continue to stabilise waste plastic, causing it to remain in the environment for longer.
Many different types of plastic exist and each may be vulnerable to several types of degradation, which usually results in several different stabilisers being used in combination. Even for objects made from the same type of plastic, different applications may have different stabilisation requirements. Regulatory considerations, such as food contact approval are also present. A wide range of stabilizers is therefore needed.
The market for antioxidant stabilisers was estimated at US$1.69 billion for 2017, with the total market for all stabilizers expected to reach US$5.5 billion by 2025.
Antioxidants
Antioxidants inhibitautoxidation
Autoxidation (sometimes auto-oxidation) refers to oxidations brought about by reactions with oxygen at normal temperatures, without the intervention of flame or electric spark. The term is usually used to describe the gradual degradation of organi ...
that occurs when polymers reacts with atmospheric oxygen. Aerobic degradation occurs gradually at room temperature, but almost all polymers are at risk of thermal-oxidation when they are processed at high temperatures. The molding or casting of plastics (e.g. injection molding
Injection moulding (U.S. spelling: injection molding) is a manufacturing process for producing parts by injecting molten material into a mould, or mold. Injection moulding can be performed with a host of materials mainly including metals (for ...
) require them to be above their melting point or glass transition temperature
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials) from a hard and relatively brittle "glassy" state into a viscous or rub ...
(~200-300 °C). Under these conditions reactions with oxygen occur much more rapidly. Once initiated, autoxidation can be autocatalytic
A single chemical reaction is said to be autocatalytic if one of the reaction products is also a catalyst for the same or a coupled reaction.Steinfeld J.I., Francisco J.S. and Hase W.L. ''Chemical Kinetics and Dynamics'' (2nd ed., Prentice-Hall 199 ...
. As such, even though efforts are usually made to reduce oxygen levels, total exclusion is often not achievable and even exceedingly low concentrations of oxygen can be sufficient to initiate degradation. Sensitivity to oxidation varies significantly depending on the polymer in question; without stabilizers polypropylene
Polypropylene (PP), also known as polypropene, is a thermoplastic polymer used in a wide variety of applications. It is produced via chain-growth polymerization from the monomer propylene.
Polypropylene
belongs to the group of polyolefins an ...
and unsaturated polymers such as rubber
Rubber, also called India rubber, latex, Amazonian rubber, ''caucho'', or ''caoutchouc'', as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds. Thailand, Malaysia, a ...
will slowly degrade at room temperature where as polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It is ...
can be stable even at high temperatures. Antioxidants are of great importance during the process stage, with long-term stability at ambient temperature increasingly being supplied by hindered amine light stabilizers (HALs). Antioxidants are often referred to as being primary or secondary depending on their mechanism of action.
Primary antioxidants (radical scavengers)
radical scavenger A scavenger in chemistry is a chemical substance added to a mixture in order to remove or de-activate impurities and unwanted reaction products, for example oxygen, to make sure that they will not cause any unfavorable reactions. Their use is wide ...
s and remove peroxy radicals (ROO•), as well as to a lesser extent alkoxy radicals (RO•), hydroxyl radical
The hydroxyl radical is the diatomic molecule . The hydroxyl radical is very stable as a dilute gas, but it decays very rapidly in the condensed phase. It is pervasive in some situations. Most notably the hydroxyl radicals are produced from the ...
s (HO•) and alkyl radicals (R•). Oxidation begins with the formation of alkyl radicals, which are formed when the high temperatures and high shear stress experienced during processing snaps the polymer chains in a homolytic manner. These alkyl radicals react very rapidly with molecular oxygen (rate constants ≈ to give peroxy radicals, which in turn abstract hydrogen from a fresh section of polymer in a chain propagation step to give new alkyl radicals. The overall process is exceedingly complex and will vary between polymers but the first few steps are shown below in general:
:R-R → 2 R•
:R• + O2 → ROO•
:ROO• + RH → ROOH + R•
Due to its rapid reaction with oxygen the scavenging of the initial alkyl radical (R•) is difficult and can only be achieved using specialised antioxidants the majority of primary antioxidants react instead with the longer lasting peroxy radicals (ROO•). Hydrogen abstraction is usually the rate determining step
In chemical kinetics, the overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step. For a given reaction mechanism, the prediction of the ...
in the polymer degradation and the peroxy radicals can be scavenged by hydrogen donation from an alternative source, namely the primary antioxidant. This converts them into an organic hydroperoxide
Hydroperoxides or peroxols are compounds containing the hydroperoxide functional group (ROOH). If the R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. O ...
(ROOH). The most important commercial stabilizers for this are hindered phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (— O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds ...
such as BHT BHT may refer to:
* Berliner Hochschule für Technik, is the second largest University of Applied Sciences in Berlin
* Black hairy tongue
* Blue Hill Troupe, a New York City-based musical theatre company
* Boğaziçi Hava Taşımacılığı, a f ...
or analogues thereof and secondary aromatic amines such as alkylated-diphenylamine
Diphenylamine is an organic compound with the formula (C6H5)2NH. The compound is a derivative of aniline, consisting of an amine bound to two phenyl groups. The compound is a colorless solid, but commercial samples are often yellow due to oxidize ...
. Amines are typically more effective, but cause pronounced discoloration, which is often undesirable (i.e., in food packaging, clothing). The overall reaction with phenols is shown below:
:ROO• + ArOH → ROOH + ArO•
:ArO• → nonradical products
The end products of these reactions are typically quinone methides, which may also impart unwanted colour. Modern phenolic antioxidants have complex molecular structures, often including a propionate-group at the para position of the phenol (i.e. they are ortho-alkylated analogues of phloretic acid). The quinone methides of these can rearrange once to give a hydroxycinnamate, regenerating the phenolic antioxidant group and allowing further radicals to be scavenged. Ultimately however, primary antioxidants are sacrificial and once they are fully consumed the polymer will being to degrade.
Secondary antioxidants (hydroperoxides scavengers)
 Secondary antioxidants act to remove
Secondary antioxidants act to remove organic hydroperoxide
In organic chemistry, organic peroxides are organic compounds containing the peroxide functional group (). If the R′ is hydrogen, the compounds are called hydroperoxides, which are discussed in that article. The O−O bond of peroxides easily ...
s (ROOH) formed by the action of primary antioxidants. Hydroperoxides are less reactive than radical species but can initiate fresh radical reactions:
:ROOH + RH → RO• + R• + H2O
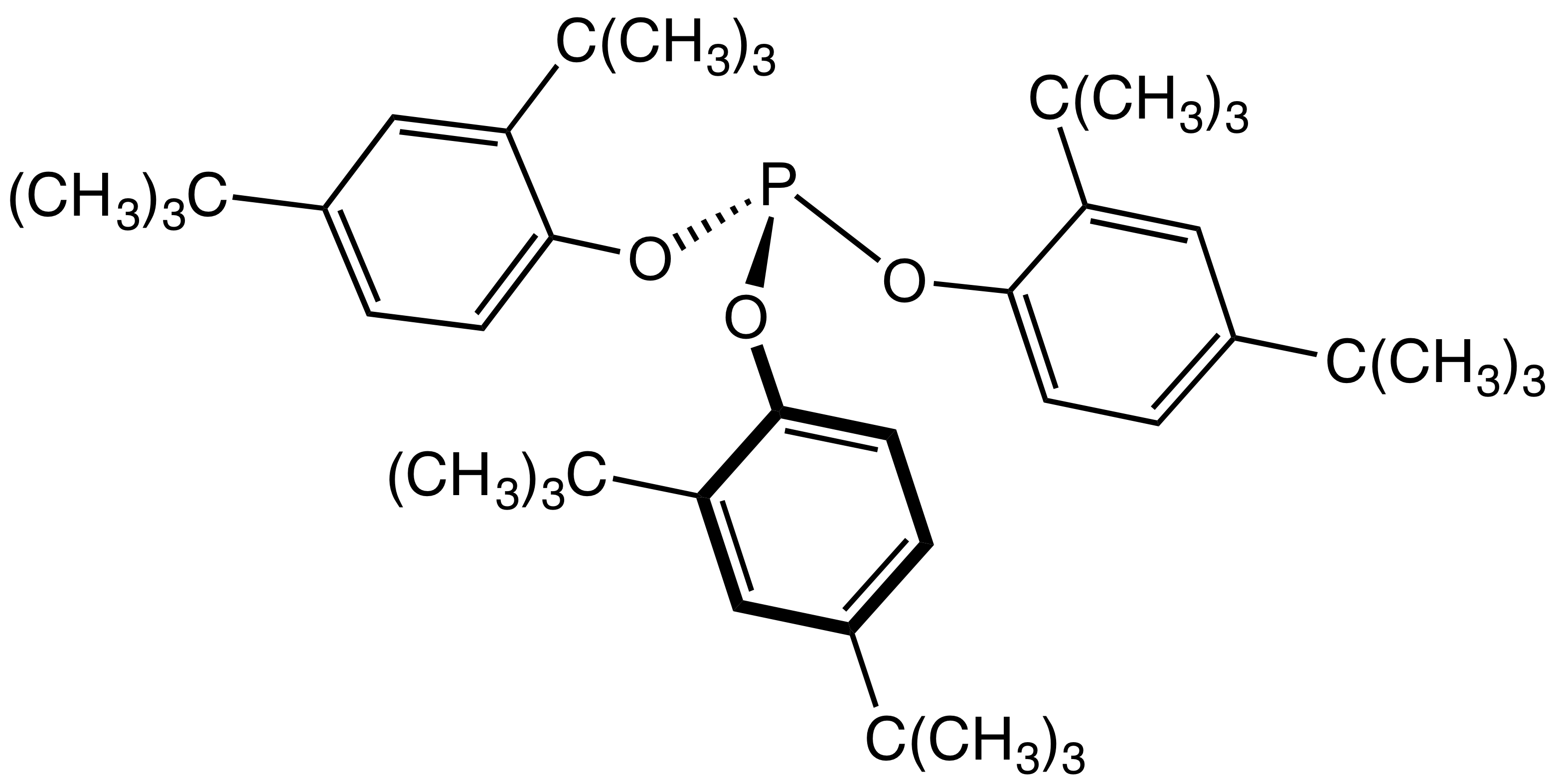
As they are less chemically active they require a more reactive antioxidant. The most commonly employed class are phosphite
The general structure of a phosphite ester showing the lone pairs on the P
In organic chemistry, a phosphite ester or organophosphite usually refers to an organophosphorous compound with the formula P(OR)3. They can be considered as esters of ...
esters, often of hindered phenols e.g. Tris(2,4-di-tert-butylphenyl)phosphite
Tris(2,4-di-tert-butylphenyl)phosphite is an organophosphorus compound with the formula C4H9)2C6H3Osub>3P. This white solid is a widely used stabilizer in polymers where it functions as an antioxidant as well as other roles. The compound is a ...
. These will convert polymer hydroperoxides to alcohols, becoming oxidized to organophosphate
In organic chemistry, organophosphates (also known as phosphate esters, or OPEs) are a class of organophosphorus compounds with the general structure , a central phosphate molecule with alkyl or aromatic substituents. They can be considered ...
s in the process:
:ROOH + P(OR')3 → OP(OR')3 + ROH
Transesterification can then take place, in which the hydroxylated polymer is exchanged for a phenol:
:ROH + OP(OR')3 → R'OH + OP(OR')2OR
This exchange further stabilizes the polymer by releasing a primary antioxidant, because of this phosphites are sometimes considered multi-functional antioxidants as they can combine both types of activity.
Organosulfur compounds
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur ...
are also efficient hydroperoxide
Hydroperoxides or peroxols are compounds containing the hydroperoxide functional group (ROOH). If the R is organic, the compounds are called organic hydroperoxides. Such compounds are a subset of organic peroxides, which have the formula ROOR. O ...
decomposers, with thioether
In organic chemistry, an organic sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A sul ...
s being particularly effective against long-term thermal aging, they are ultimately oxidise up to sulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. E ...
s and sulfone
In organic chemistry, a sulfone is a organosulfur compound containing a sulfonyl () functional group attached to two carbon atoms. The central hexavalent sulfur atom is double-bonded to each of two oxygen atoms and has a single bond to each of ...
s.
Antiozonant
ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lo ...
. This is naturally present in the air at very low concentrations but is exceedingly reactive, particularly towards unsaturated polymers such as rubber, where it causes ozone cracking
Cracks can be formed in many different elastomers by ozone attack, and the characteristic form of attack of vulnerable rubbers is known as ozone cracking. The problem was formerly very common, especially in tires, but is now rarely seen in those ...
. The mechanism of ozonolysis
In organic chemistry, ozonolysis is an organic reaction where the unsaturated bonds of alkenes (), alkynes (), or azo compounds () are cleaved with ozone (). Alkenes and alkynes form organic compounds in which the multiple carbon–carbon bon ...
is different from other forms of oxidation and hence requires its own class of antioxidant stabilizers.
These are primarily based on p-phenylenediamine
''p''-Phenylenediamine (PPD) is an organic compound with the formula C6H4(NH2)2. This derivative of aniline is a white solid, but samples can darken due to air oxidation. It is mainly used as a component of engineering polymers and composites lik ...
and work by reacting with ozone
Ozone (), or trioxygen, is an inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lo ...
faster than it can react with vulnerable functional groups in the polymer (typically alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
groups). They achieve this by having a low ionization energy
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive Electric charge, charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged a ...
which allows them to react with ozone via electron transfer, this converts them into radical cations that are stabilized by aromaticity
In chemistry, aromaticity is a chemical property of cyclic (ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to sat ...
. Such species remain reactive and will react further, giving products such as 1,4-benzoquinone
1,4-Benzoquinone, commonly known as ''para''-quinone, is a chemical compound with the formula C6H4O2. In a pure state, it forms bright-yellow crystals with a characteristic irritating odor, resembling that of chlorine, bleach, and hot plastic ...
, phenylenediamine-dimers and aminoxyl radicals. Some of these products can then be scavenged by antioxidants.
Light stabilizers
autoxidation
Autoxidation (sometimes auto-oxidation) refers to oxidations brought about by reactions with oxygen at normal temperatures, without the intervention of flame or electric spark. The term is usually used to describe the gradual degradation of organi ...
this is a free radical process, hence the antioxidants described above are effective inhibiting agents, however additional classes of additives are also beneficial, such as UV absorbers, quenchers of excited states and HALS.
UV absorbers
UV susceptibility varies significantly between different polymers. Certainpolycarbonate
Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, tough materials, and some grades are optically transparent. They are easily work ...
s, polyesters and polyurethane
Polyurethane (; often abbreviated PUR and PU) refers to a class of polymers composed of organic chemistry, organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethan ...
s are highly susceptible, degrading via a Photo-Fries rearrangement. UV stabilisers absorb and dissipate the energy from UV rays as heat, typically by reversible intramolecular proton transfer. This reduces the absorption of UV rays by the polymer matrix and hence reduces the rate of weathering. Benzotriazole
Benzotriazole (BTA) is a heterocyclic compound with the chemical formula C6H5N3. Its five-membered ring contains three consecutive nitrogen atoms. This bicyclic compound may be viewed as fused rings of the aromatic compounds benzene and triazole. ...
s and hydroxyphenyl-triazines (like Bemotrizinol) are used to stabilise polycarbonate
Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, tough materials, and some grades are optically transparent. They are easily work ...
s and acrylic
Acrylic may refer to:
Chemicals and materials
* Acrylic acid, the simplest acrylic compound
* Acrylate polymer, a group of polymers (plastics) noted for transparency and elasticity
* Acrylic resin, a group of related thermoplastic or thermosett ...
s, oxanilides are used for polyamide
A polyamide is a polymer with repeating units linked by amide bonds.
Polyamides occur both naturally and artificially. Examples of naturally occurring polyamides are proteins, such as wool and silk. Artificially made polyamides can be made throug ...
s and polyurethanes, while benzophenone
Benzophenone is the organic compound with the formula (C6H5)2CO, generally abbreviated Ph2CO. It is a white solid that is soluble in organic solvents. Benzophenone is a widely used building block in organic chemistry, being the parent diarylke ...
s are used for PVC.
Strongly light-absorbing PPS is difficult to stabilize. Even antioxidants fail in this electron-rich polymer. The acids or bases in the PPS matrix can disrupt the performance of the conventional UV absorbers such as HPBT. PTHPBT, which is a modification of HPBT are shown to be effective, even in these conditions.
Quenchers
chromophore
A chromophore is the part of a molecule responsible for its color.
The color that is seen by our eyes is the one not absorbed by the reflecting object within a certain wavelength spectrum of visible light. The chromophore is a region in the molec ...
within the polymer (which may be a dye or impurity) causing it to enter an excited state
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers t ...
. This can then react with ambient oxygen, converting it into highly reactive singlet oxygen
Singlet oxygen, systematically named dioxygen(singlet) and dioxidene, is a gaseous inorganic chemical with the formula O=O (also written as or ), which is in a quantum state where all electrons are spin paired. It is kinetically unstable at ambie ...
. Quenchers are able to absorb energy from excited molecules via a Förster Förster or Foerster is a German surname meaning " forester". (It has often been Anglicised as Forster). Notable people of this name include:
Förster
* Arnold Förster (1810–1884), a German entomologist
* August Förster (physician) (1822–1 ...
mechanism and then dissipate it harmlessly as either heat or lower frequency fluorescent light. Singlet oxygen can be quenched by metal chelates, with nickel phenolates being a common example.
Hindered amine light stabilizers
polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging (plastic bags, plastic films, geomembranes and containers including ...
and polyurethane
Polyurethane (; often abbreviated PUR and PU) refers to a class of polymers composed of organic chemistry, organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethan ...
, they are ineffective in polyvinyl chloride (PVC). It is thought that their ability to form nitroxyl radicals is disrupted. HALS act as a base and become neutralized by hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the dig ...
(HCl) that is released by photooxidation of PVC. The exception is the recently developed NOR HALS, which is not a strong base and is not deactivated by HCl.
Other Classes
Polymers are susceptible to degradation by a variety of pathways beyond oxygen and light.Acid Scavengers
Acid scavengers, also referred to as antacids, neutralize acidic impurities, especially those that releaseHCl HCL may refer to:
Science and medicine
* Hairy cell leukemia, an uncommon and slowly progressing B cell leukemia
* Harvard Cyclotron Laboratory, from 1961 to 2002, a proton accelerator used for research and development
* Hollow-cathode lamp, a s ...
. PVC is susceptible to acid-catalyzed degradation, the HCl being derived from the polymer itself. Ziegler–Natta catalyst A Ziegler–Natta catalyst, named after Karl Ziegler and Giulio Natta, is a catalyst used in the synthesis of polymers of 1-alkenes ( alpha-olefins). Two broad classes of Ziegler–Natta catalysts are employed, distinguished by their solubility:
* ...
s and halogenated flame retardants also serve as sources of acids. Common acid scavengers include metallic soaps, such as calcium stearate and zinc stearate, mineral agents, such as hydrotalcite
Hydrotalcite or formerly also Völknerite is a layered double hydroxide (LDH) of general formula ·4, whose name is derived from its resemblance with talc and its high water content. Multiple structures containing loosely bound carbonate ions exi ...
and hydrocalumite, and basic metal oxides, such as calcium oxide
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term "'' lime''" connotes calcium-containing inorganic ...
, zinc oxide
Zinc oxide is an inorganic compound with the Chemical formula, formula . It is a white powder that is insoluble in water. ZnO is used as an additive in numerous materials and products including cosmetics, food supplements, rubbers, plastics, ceram ...
or magnesium oxide
Magnesium oxide ( Mg O), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ...
.
Metal deactivators
Metal ions, such as those of Ti, Al and Cu, can accelerate the degradation of polymers. This is of particular concern where polymers are in direct contact with metal, such as in wiring and cable. More generally, the metal catalysts used to form the polymer may simply become encapsulated within it during production, this is typically true of Ziegler-Natta catalysts inpolypropylene
Polypropylene (PP), also known as polypropene, is a thermoplastic polymer used in a wide variety of applications. It is produced via chain-growth polymerization from the monomer propylene.
Polypropylene
belongs to the group of polyolefins an ...
. In these instances metal deactivator
Metal deactivators, or metal deactivating agents (MDA) are fuel additives and oil additives used to stabilize fluids by deactivating (usually by sequestering) metal ions, mostly introduced by the action of naturally occurring acids in the fuel and ...
s may be added to improve stability. Deactivators work by chelation
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are ...
to form an inactive coordination complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
with the metal ion. Salen-type compounds are common.
Heat stabilizers
Heat (or thermal) stabilizers are mostly used for PVC, as unstabilized material is particularly prone to thermal degradation. These agents minimize loss of HCl, a degradation process that starts above 70 °C. Once dehydrochlorination starts, it isautocatalytic
A single chemical reaction is said to be autocatalytic if one of the reaction products is also a catalyst for the same or a coupled reaction.Steinfeld J.I., Francisco J.S. and Hase W.L. ''Chemical Kinetics and Dynamics'' (2nd ed., Prentice-Hall 199 ...
. Many diverse agents have been used including, traditionally, derivatives of heavy metals (lead, cadmium). Increasingly, metallic soaps (metal "salts" of fatty acid
In chemistry, particularly in biochemistry, a fatty acid is a carboxylic acid with an aliphatic chain, which is either saturated or unsaturated. Most naturally occurring fatty acids have an unbranched chain of an even number of carbon atoms, ...
s) are favored, species such as calcium stearate.M. W. Allsopp, G. Vianello, "Poly(Vinyl Chloride)" in ''Ullmann's Encyclopedia of Industrial Chemistry'', 2012, Wiley-VCH, Weinheim. .
Addition levels vary typically from 2% to 4%.
The choice of the best heat stabilizer depends on its cost effectiveness in the end use application, performance specification requirements, processing technology and regulatory approvals.
Flame retardants
Flame retardants are a broad range of compounds that improve fire resistance of polymers. Examples include brominated compounds along withaluminium hydroxide
Aluminium hydroxide, Al(OH)3, is found in nature as the mineral gibbsite (also known as hydrargillite) and its three much rarer Polymorphism (materials science), polymorphs: bayerite, doyleite, and nordstrandite. Aluminium hydroxide is Amphoter ...
, antimony trioxide
Antimony(III) oxide is the inorganic compound with the formula Sb2O3. It is the most important commercial compound of antimony. It is found in nature as the minerals valentinite and senarmontite. Like most polymeric oxides, Sb2O3 dissolves i ...
, and various organophosphate
In organic chemistry, organophosphates (also known as phosphate esters, or OPEs) are a class of organophosphorus compounds with the general structure , a central phosphate molecule with alkyl or aromatic substituents. They can be considered ...
s. Flame retardants are known to reduce the effectiveness of antioxidants.
Biocides
Degradation resulting from microorganisms (biodegradation
Biodegradation is the breakdown of organic matter by microorganisms, such as bacteria and fungi. It is generally assumed to be a natural process, which differentiates it from composting. Composting is a human-driven process in which biodegrad ...
) involves its own class of special bio-stabilizers and biocide
A biocide is defined in the European legislation as a chemical substance or microorganism intended to destroy, deter, render harmless, or exert a controlling effect on any harmful organism. The US Environmental Protection Agency (EPA) uses a slig ...
s (e.g. isothiazolinone
Isothiazolinone (sometimes isothiazolone) is an organic compound with the formula (CH)2SN(H)CO. A white solid, it is structurally related to isothiazole. Isothiazolone itself is of limited interest, but several of its derivatives are widely used ...
s).
See also
*Oil additive
Oil additives are chemical compounds that improve the lubricant performance of base oil (or oil "base stock"). The manufacturer of many different oils can utilize the same base stock for each formulation and can choose different additives for each ...
s and fuel additive
Petrol additives increase petrol's octane rating or act as corrosion inhibitors or lubricants, thus allowing the use of higher compression ratios for greater efficiency and power. Types of additives include metal deactivators, corrosion inhibitors, ...
s often include antioxidant stabilizers related to the ones discussed in this article
* Polymer degradation
Polymer degradation is the reduction in the physical properties of a polymer, such as strength, caused by changes in its chemical composition. Polymers and particularly plastics are subject to degradation at all stages of their product life cycl ...
, polymer weathering and environmental stress cracking
Environmental Stress Cracking (ESC) is one of the most common causes of unexpected brittle failure of thermoplastic (especially amorphous) polymers known at present. According to ASTM D883, stress cracking is defined as "an external or intern ...
- discuss the natural degradation of polymers
* Chemically Assisted Degradation of Polymers
Polymer degradation is the reduction in the physical properties of a polymer, such as strength, caused by changes in its chemical composition. Polymers and particularly plastics are subject to degradation at all stages of their product life cycle ...
and weather testing of polymers - discuss the accelerated degradation of polymers
* Biodegradable additives
Biodegradable additives are additives that enhance the biodegradation of polymers by allowing microorganisms to utilize the carbon within the polymer chain as a source of energy. Biodegradable additives attract microorganisms to the polymer throug ...
- are additives that enhance the biodegradation of polymers
;Other additives
* Plasticizer
A plasticizer ( UK: plasticiser) is a substance that is added to a material to make it softer and more flexible, to increase its plasticity, to decrease its viscosity, and/or to decrease friction during its handling in manufacture.
Plasticiz ...
* Filler (materials)
Filler materials are particles added to resin or binders (plastics, composites, concrete) that can improve specific properties, make the product cheaper, or a mixture of both. The two largest segments for filler material use is elastomers and pla ...
* Plastic colorants
* Mold release agents
References
{{plastics Plastics additives Material protection